
Concept explainers
Write structural formulas for each of the following alcohols and
(a)
(e)
(b)
(f)
(c)
(g)
(d)
(h)
Interpretation:
The structural formulas for the given alcohols and alkyl halides are to be written.
Concept introduction:
While making the structure of an alcohol, the name of the parent alcohol is used to determine the number of atoms in the parent chain.
The substituents attached and their positions on the parent chain are determined from the name and these are drawn accordingly.
Solid lines are used to show the covalent bonds between atoms.
The structural formula of a compound shows how the atoms are arranged and bonded to each other in the compound.
Answer to Problem 19P
Solution:
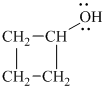
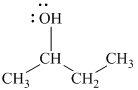

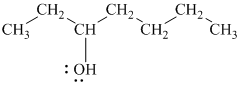
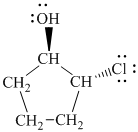
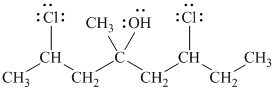
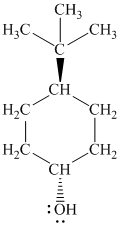
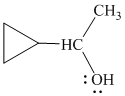
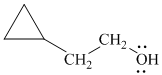
Explanation of Solution
a)
In
Therefore, the structure of
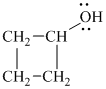
b)
In
The structure of
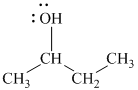
c)
In
Therefore, the structure of
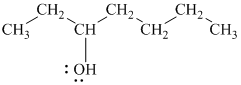
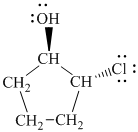
d)
In
The prefix ‘trans’ indicates that the chlorine and hydroxyl groups are on the opposite sides of the cyclopentane ring.
The structure of
e)
In
Therefore, the structure of
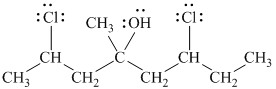
f)
In
The structure of
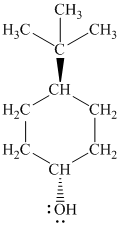
g)
In
The structure of
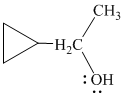
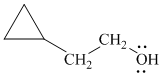
h)
In
The structure of
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- Formaldehyde, H2C=O, is known to all biologists because of its usefulness as a tissue preservative. When pure, formaldehyde trimerizes to give trioxane, C3H6O3, which, surprisingly enough, has no carbonyl groups. Only one monobromo derivative (C3H5BrO3) of trioxane is possible. Propose a structure for trioxane.arrow_forwardIdentify the oxygen-containing functional groups in these small molecules: .H Н н Н C C -H HF 3HHH H-C -H Н Н aldehyde Н Н ether alcohol ester ketone aldehyde carboxylic acid carboxylic acid ester alcohol ether ketonearrow_forwardWrite the systematic name of each organic molecule: structure OH H-C-CH-CH-CH₂-OH I CH3 CH3 I CH,—CH–CH2−CH O || H—C–CH–CH3 I CH3 name 0 0 Xarrow_forward
- 12 What type of organic molecule is this? нн | н-с-с-с H H O-H ketone O alcohol aldehyde organic acidarrow_forwardWhich of the following ions are aromatic species? II II IV O Ill and IV O l and II O Il and IV O Il and IIarrow_forwarda Complete the following reaction of a disulfide with a reducing agent. What is the structure of the organic product (of which two moles are formed)? CH3CH2-S-S-CH2CH3 + 2(H) · C P орy aste CH4 ChemDoodle®arrow_forward
- Name the following compounds: ÇH3 (a) CH;CH,CH,-CH CH3 (b) CH,CH-CHҪH—С—СH ČH,CH,CH3 Н Нarrow_forwardBelow is a structural formula. What is the corresponding condensed structural H formula? H1C1I Н Н OCH3NHC(CH3)2 H-CN-C-C CH3NHCH(CH3)2 OCH3NC(CH3)2 :Z―I OCH3NCH(CH3)2 H H H I— H -C-H H (arrow_forwardWrite the systematic name of each organic molecule: OH 1 structure CH3-CH-CH₂-CH₂-CH₂-OH OH | CH3 CH₂-CH-CH₂-CH3 OH HỌ—CH, — CH–CH2–CH2–CH3 name 7 0 0 X Sarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





