
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
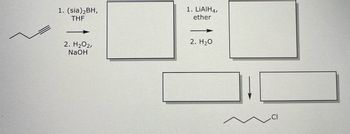
Transcribed Image Text:1. (sia) ₂BH,
THF
2. H₂O2,
NaOH
1. LiAlH4,
ether
2. H₂O

Transcribed Image Text:Mg
ether
+
:0: H30+
ОН
у час
н
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sulfuric acid (H₂SO₂) reacts with sodium bicarbonate (NaHCO3) to produce sodium sulfate, carbon dioxide and water. If 2.48 L of 3.00 M H₂SO is spilled and 1.25 kg NaHCO3 (MM=84.01 g/mol) is available to clean up the spill, is there enough sodium bicarbonate to neutralize the spilled sulfuric acid? A. ○ Yes, and there is about 20% more NaHCO, than needed. B. Yes, and there is about 10% more NaHCO than needed. C. O No, and there is about 20% less NaHCO₂ than needed. D. No, and there is about 10% less NaHCO3 than needed. E. Yes, and there is precisely enough NaHCO₂.arrow_forwardBalance the following equation. Be sure to place a number in front of each molecule. Use "1"s if needed. 3 Na2O + 1 CaO + 2 Ca3(PO4)2 Na3PO4 <-- 3arrow_forwardWrite an equation to show that hydrochloric acid , HCl , behaves as an acid in water. Write an equation to show that hypochlorous acid , HClO , behaves as an acid in water. Write a net ionic equation to show that phosphoric acid, H3PO4, behaves as an acid in water. Consider only its first ionization.arrow_forward
- Antacid tablets are used to neutralize stomach acid and relieve indigestion or acid reflux. Many commercial antacids contain magnesium hydroxide, Mg(OH)2, as the active ingredient. If one tablet of antacid contains 15 mg of Mg(OH)2, then calculate the number of tablets required to completely neutralize 5 mL of stomach acid if the stomach acid is 0.2 M HCl.arrow_forwardENTS IN GENERAL CHEMISTRY GENERAL CHEMISTRY STUDY SHEET NO. 4 1. Balance the following chemical equations: a) C„H; + O, → CO2 + H,O s b) Al,S, + PbCl,4 PbS, + A1CI, nos 0 c) BaCl, + Na,PO, → Ba, (PO), + NaCl 2. Assume the follon 100arrow_forward1. (Balance the equation) H,SO+NaOH>H,SO+H,Oarrow_forward
- 3. Pure (glacial) acetic acid doesn't conduct electricity while 1.0 M aqueous acetic acid does, somewhat. Write an equation to show how acetic acid reacts with water to produce ions. (One of those ions is the hydrated H* ion, written as H30*.)arrow_forward2+ Natural waters often contain relatively high levels of calcium ion, Ca²+, and hydrogen carbonate ion (bicarbonate), HCO3¯, from the leaching of minerals into the water. When such water is used commercially or in the home, heating of the water leads to the formation of solid calcium carbonate, CaCO3, which forms a deposit ("scale") on the interior of boilers, pipes, and other plumbing fixtures. Ca(HCO3)2 (aq) → CaCO3 (s) + CO2(g) + H₂O(1) If a sample of well water contains 0.0016 mg of Ca(HCO3)2 per milliliter, what mass of CaCO3 scale would 1.0 mL of this water be capable of depositing? Mass= garrow_forwardMagic Acid® is the commercial name for a 1:1 mixture of fluorosulfonic acid, HSO3F, and antimony pentafluoride, SbF5, that generates H2SO3F+ cations – this last species qualifies as a superacid. Can Magic Acid® be diluted in water and retain its superacid property? Justify your answer and include at least one chemical equation.arrow_forward
- Ca* OH OH Ca* Ca* Ca* OH OH OH Ca* OH OH Ca OH OH Ca** OH Ca* 2+ Ca(OH) OH Саон A C Which beaker best depicts calcium hydroxide in a beaker of water? ов O A O D O Earrow_forwardAqueous solutions of ammonia 1NH32 and bleach (active ingredient NaOCl) are sold as cleaning fluids, but bottles of both of them warn: “Never mix ammonia and bleach, as toxic gases may be produced.” One of the toxic gases that can be produced is chloroamine, NH2Cl.Another toxic gas that can be produced is nitrogen trichloride, NCl3. What is the oxidation number of N in nitrogen trichloride?arrow_forwardwrite a balanced chemical reaction for the reaction of vinegar, CH3COOH(aq) and baking soda, NaHCO3(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY