
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
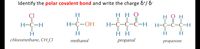
Transcribed Image Text:Identify the polar covalent bond and write the charge &+/ &-
нно
T T |
Н-С-Ҫ-С-Н
ÇI
нон
H-C-H
Н-С-ОН
Н-С-С-С-Н
нн
chloromethane, CH,CI
methanol
propanal
propanone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't give handwritten answers please oarrow_forward16) Which of the following molecules are isomers? Η Η Η H-C-C-C-OH Η Η Η II Η Η Η Η T H-C-C-C-C-OH | | Η Η Η Η I όλο το I, II and III I and II I and III II and III none of the molecules are isomers Η Η ΟΗΗ |||| H-C-C-C-C-H I Η Η Η Η ΙΠarrow_forwardNaming branched alkanes Name the following organic compounds: compound CH3 — CH₂' - CH3- - CH₂ C | CH₂- CH - - - - CH2- - CH3 - CH2 CH2 — CH3 CH3 CH₁₂ CH name ☐ - CH- - CH2-CH2- CH3 ☐ CH3 CH2-CH3 - CH3 | CH, CH3 CH - CH3 ☐ اه Aarrow_forward
- * CON|OU|GR|OC|G app.101edu.co Tube Maps > A 2 W S X # C|OC|OU XGdD Question 30 of 31 Provide the correct IUPAC name for the skeletal (line-bond) structure shown here. Br 3- 4- 2- 5- 1- cyclo tert- tri sec- di ISO pent chloro prop MacBook Air F6 3 II 80 F3 E D C > $ < F4 R ♂ Aav F V P₂ do LO 5 % P F5 T B G B < C 6 B Y H H|2|GA|GV| |bs|G₁1CSUONEE & 7 N 6- 6 F7 U * 8 J DII F8 M ( 9 K 8 F9 0 0 L F10 P Done 1 B Gr. F11 + { [ 中華 F12 + } 1arrow_forwardJ C @ 2 F2 W S What is the correct IUPAC name for the compound shown here? CO # 3 F3 e d F4 $ A ܠ 4 f F5 % 5 3- t Question 37 of 49 ISO 9 1- M F6 A 6 5- tert- neo di 2- 6- 4- sec- O DELL y h F7 & 7 F8 j * 8 F9 k F10 0 ) F11 Р F12 Mar 24 JE L + 11 8 deletearrow_forwardDraw the Lewis structure of the missing reactant. Make sure to include lone pairs and non-zero formal charges. OH + ? - H,O + CH,CH,O Click and drag to start drawing a structure. 5 Carrow_forward
- LYRICA (pregabalin) is an oral pharmaceutical primarily indicated for the management of fibromyalgia and neuropathic pain. At a pH of 11, LYRICA has an overall charge of –1, with most atoms neutral and only one oxygen showing a –1 formal charge. The structure for LYRICA at pH 11 is shown below. Add all the missing lone pairs to the given structure of LYRICA.arrow_forwardWhich of the following structures shows the correct arrow pushing for a valid resonance structure of the following compound? H2N. OD ОА Ов OC A 앵 H₂N- H₂N- Б OH (он ОН най OH H₂N C OH OH H₂N OH В OHarrow_forwardThe carbon skeleton for 2-methylpropane is shown below. How many hydrogens are present? c-d-c 4 9 10 13 14 16arrow_forward
- Write the common (not systematic) name of each organic molecule. structure CH3 CH₂ CH3 1 CH3-CH-N-CH-CH3 - | CH3 CH3-NH2 CH3 CH3-CH₂-CH₂-N-CH₂ - CH₂ - CH3 name 0 0 0 X Śarrow_forwardUsing your general knowledge of periodic trends in electronegativity, predict whether the C=O bond and the C-H bonds in formaldehyde will be strongly polar, weakly polar or nonpolar. Calculate the electronegativity difference ΔχO-C across the C=O bond and the electronegativity difference ΔχC-H to confirm or correct your predictions. For any strongly polar bond(s) on the molecule, draw the bond dipole moment adjacent to the bond in the VSEPR structure above, using crossed arrow notation to show the direction of the positive and negative bond dipoles. Predict whether the overall molecule should be polar or nonpolar. ___________________ Explain your reasoning below. If the overall molecule is polar, draw the net dipole next to the molecule, using crossed arrow notation to indicate the direction of the dipole.arrow_forward-ОН ACO OAc -ОАс оarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY