
Concept explainers
Interpretation:

The IUPAC names of the given compounds are to be determined.
Concept introduction:
The halogen is treated as substituent on an
The two substituents are considered of equal rank, when both halogen and an alkyl substituent are on the same carbon chain. The substituent nearer the end of the chain is given the lower number.
Answer to Problem 20P
Solution:
Explanation of Solution
(a)
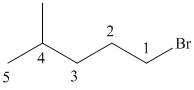
In this structure, the longest carbon chain is “pentane,” which contains
Therefore, the IUPAC name of the given structure is
(b)
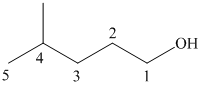
In this structure, the longest carbon chain is “pentane,” which contains
Therefore, the IUPAC name of given structure is
(c)
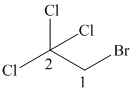
In this structure, the longest carbon chain is “ethane,” which contains
Therefore, the IUPAC name of given structure is
(d)
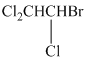
The given compound is converted to the structural formula as shown below:
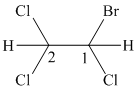
In the given structure, the longest possible parent chain is “ethane,” which contains
Therefore, the IUPAC name of given structure is
(e)
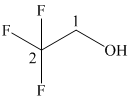
In the given structure, the longest possible parent chain is “ethane,” which contains
(f)
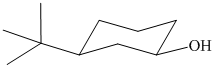
In the given structure, the parent chain is cyclic and has six carbon atoms. Hence the parent alkane is cyclohexane. One carbon atom is attached to a hydroxyl group. Hydroxyl groups take precedence over alkyl groups in determining the direction in which a carbon chain is numbered. Hence, the carbon atom attached to the hydroxyl group is numbered
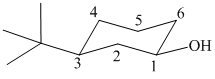
Thus, the IUPAC name of the compound is
(g)
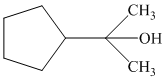
In the given structure, a five member cyclopentane ring is present. There is a three carbon atom chain bearing the hydroxyl group. The propane chain becomes the parent since the hydroxyl group is attached to it. The cyclopentane ring is considered as substituent and attached to the second carbon of the propane chain as shown:
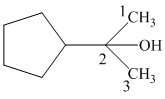
Thus, the IUPAC name of the given structure is
(h)
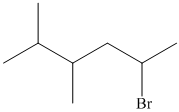
In the given structure, the longest possible parent chain is “Hexane,” which contains
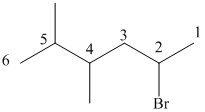
The prefix di is used to indicate the presence of two methyl groups.
Thus, the IUPAC name of the given structure is
(i)
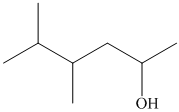
In the given structure, the longest possible parent chain is “Hexane,” which contains
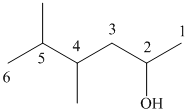
Thus, the IUPAC name of the compound is
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- OH OH (1) NaOH - (x) (2) H® Reactant x is : 7. (a) CH3CI (b) CH 2C12 (c) CHCI3 (d) CCI4arrow_forwardAssign an IUPAC name to each of the following unsaturated hydrocarbons. H3C-C=CH₂ H (a) (b) (c) (d) H H3C-C-C-C-CH3 CH3 CH3 H & propene 2-butynearrow_forwardGive the IUPAC names of the following alcohols. CH, HO OH (а) `CH, (b) Br ỌH CH3 (с) (d) OH ČH, ÇH,CH,CH, H;C c=C CH,CH, CI H- Но- (е) (f) H- -Br CH,OH ČH,arrow_forward
- For each of the following pairs, determine if the compounds have the same boiling point or different boiling points. (a) (b) OH OH (c) CH,OH CH,OH H- OH H- HO H -O- HO -H OH H- OH H -O- CH2OH CH2OH (d) OH OH (e) OH OH OH OHarrow_forwardDraw an expanded structural formula of pent-1-en-3-yne/ CH3-CC-CH=CH2 and then label each carbon. Indicate the longest and shortest C-H bond and predict the C—C single bond that has the highest BDE(bond dissociation energy).arrow_forwardThe action of heating one mole of water (containing a trace of acid catalyst) on one mole of compound Y produces one mole of CH,COOH and one mole of CH, CH,NH, The structure of Y is: Select one: O CHCNCCH, CH,CH,NHCH,CH, OH CH CHNHCH,CH, O CHCH,CNHCH; O CH;CH NHÖCH;arrow_forward
- ► < (b) H₂C B B W Chapter... 135 Provide the systematic (IUPAC) name for each of the following saturated hydrocarbons. (a) H₂ H₂C- (c) H₂C H₂C H₂ H₂ -C H₂C H3C CH₂ H₂C H₂C CH₂ H₂ HC CH3 CH₂ H₂ H₂ CH₂C H₂ CH₂ CH₂ H₂C CH₂ CH G + webassign.net C S E MacBook Air Garrow_forwardHgSO4 H2SO4, H20 1T (1) Br H2O2, NaOH 10 NaNH2 BH3, THE H =H 1S NH3 H20 HCI (1 equiv.) diethyl ether 1V 1W Br2 (m) MgBr (H,C=CH),CULI NaOCI ТСРВА > 1X 1Y > 1Z CH3COOH 1AA diethyl ether SoCI2, pyridine diethyl ether 1ABarrow_forwardArrange these compounds in order of increasing boiling point (values in C are 42, 24, 78, and 118). (a) CH3CH2OH (b) CH3OCH3 (c) CH3CH2CH3 (d) CH3COOHarrow_forward
- app.101edu.co uTube Maps 35 80 F2 F3 F4 ! 1 ion Q A L N 2 W S X H 5 command #3 E D C Be dc Ho [S Ma Question 32 of 48 What is the correct IUPAC name for the compound below? $ 4 R F do 5 % V F5 -4 T X G D 6 MacBook Air F6 Y 7 F7 U H BN W * A) 3-propyl-4-ethylheptane B) isooctane C) 3,4-dipropylhexane D) 4,5-diethyloctane E) None of these are correct ▷▷ F9 DII F8 8 T J M ( 9 HG 17 C Sc UO NO K ) O L H 7 L F10 [ {T A ? 1 command option Et P F11 + 11 WL Baarrow_forwardPredict reagents needed to complete this E1 elimination reaction. Brarrow_forwardExample: H R 11' COO- Ca ↓ NH3 L or D?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


