
(a)
Interpretation:
The systematic name for the following compound should be determined.
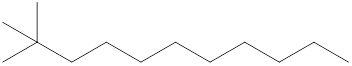
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(b)
Interpretation:
The systematic name for the following compound should be determined.
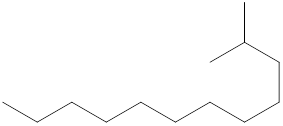
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(c)
Interpretation:
The systematic name for the following compound should be determined.
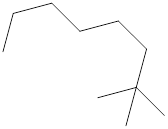
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(d)
Interpretation:
The systematic name for the following compound should be determined.
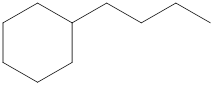
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which a series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and their atoms don't form a ring is known as acyclic compounds. The general molecular formula of a cyclic alkane is
Rules of naming cycloalkanes are:
- First, determine the cycloalkane present in the structure which is considered as a parent chain (maximum number of carbon atoms). If the acyclic alkane chain has more carbon atoms, then the alkyl chain is considered a parent chain.
- For a cyclic system, the number of carbon atoms must be identified as present in different paths connected with two bridgeheads.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group or cycloalkyl group and denote its position on the parent chain with the number
- The alkyl groups or cycloalkyl groups are written in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Ethylene diamine (bp 116 °C) and water form a maximum boiling azeotrope (bp 120 °C); the azeotropic mixture is 80% in ethylene diamine. SKETCH a distillation curve (volume on the x-axis and temperature on the y-axis) to show what you would expect to see should one distill 35 mL of a 50:50 mixture of these liquids. Be as quantitatively accurate as possible (using graph paper).arrow_forward3. When solid sodium bicarbonate and solid hydrochloric acid were mixed together, no change was observed. When water was added to the solid mixture, bubbles formed. Explain these results.arrow_forward1 4 7 8 When 2.19 g of a certain molecular compound X are dissolved in 100. g of formamide (NH, COH), the freezing point of the solution is measured to be - 0.4 °C. Calculate the molar mass of X. If you need any additional information on formamide, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. Submit Assign Continue O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accesarrow_forward
- Consider the reaction of a 20.0 mL of 0.220 M C₅H₅NHCl (Ka = 5.9 x 10⁻⁶) with 12.0 mL of 0.219 M CsOH. Write the net ionic equation for the reaction that takes place. Be sure to include the proper phases for all species within the reaction.arrow_forward5.0 mL of 1-butanol was mixed with 10 mL of HCl. The reaction was put on an ice bath and 4 mL of concentrated sulfuric acid was added. This mixture was refluxed for 45 min and then the product was co-distilled with water using a simple distillation apparatus. Water and the product are immiscible. 1-butanol has a molar mass of 74.12 g/mol, a density of 0.810 g/mL, and a boiling point of 118 C. The product has a molar mass of 92.57 g/mol, a density of 0.880 g/mL, and a boiling point of 78 C. What is the nucleophile in this reaction? What is the role of H2SO4 in this reaction? Calculate the theoretical yield for this reaction. Give your answer in grams. Select the following statements that can be said about the reaction shown.arrow_forwardWhat is the freezing point of a solution containing 3.10 grams benzene (molar mass = 78.11 g mol-1) dissolved in 32.0 grams of paradichlorobenzene (molar mass = 147.0 g mol-1)? The freezing point of pure paradichlorobenzene is 53.0 °C, and the freezing point depression constant is 7.10 K kg mol-1.arrow_forward
- You have a 5 mL sample of a protein in 0.5 M NaCl. You place the protein/salt sample inside dialysis tubing (see Fig.) and place the bag in a large beaker of distilled water. If your goal is to remove as much NaCl from the sample as possible, which would be more eff ective: (1) placing the dialysis bag in 4 L of distilled water for 12 h, or (2) placing the bag in 1 L of distilled water for 6 h and then in another 1 L of fresh distilled water for another 6 h?arrow_forward5- A chemist is given a nonelectrolyte white powder for analysis. 38.7 g of this substance is dissolved in 100.0 g of water. It is determined that the resulting solution freezes at -4.0°C. Additionally, the elemental composition of the substance is identified and found to be 40.0% C, 6.7% H and 53.3 % O by mass. Find the molecular formula of this unknown white powder using these experimental evidences (Kş of water: 1.86°C/m, C: 12 g/mol, H: 1 g/mol, O: 16 g/mol).arrow_forwardSupposed you are in a chemistry laboratory and your CHEF102B teacher gave you a 500 ml Erlenmeyer flask containing 200 ml of heterogenous liquid mixture. This liquid mixture consists of sodium chloride, water, benzoic acid, is made by combining Mixture A and Mixture B. Mixture A (aqueous mixture): sodium chloride is dissolved in distilled water. Mixture B (organic mixture): benzoic acid, phenol and aniline is dissolved in petroleum ether (a nonpolar solvent). Your task is to separate the components of the heterogenous mixture and identify the components.arrow_forward
- Water is the solvent of choice to separate a mixture of adipic acid and salicylic acid because it dissolves both substances easily. Group of answer choices True Falsearrow_forwardA geochemist measures the concentration of salt dissolved in Lake Parsons and finds a concentration of 43.33 g L.. The geochemist also measures the concentration of salt in several nearby non-isolated lakes, and finds an average concentration of 3.1 g.1.¹. Assuming the salt concentration in Lake Parsons before it became isolated was equal to the average salt concentration in nearby non-isolated lakes, calculate the percentage of Lake Parsons which has evaporated since it became isolated. Round each of your answers to 2 significant digits. 0% 0.8 X 5arrow_forwardWhat is the solubility of sodium sulphate in water at 30°Carrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
