
Interpretation:
The given conformations are needed to be ranked in order of increasing energy.
Concept introduction:
Conformations are arising due to the free rotation of
The most and least energy occupied conformations are eclipsed and staggered, which are having a dihedral of ‘0’ and ‘60’ degree respectively.
Staggered conformation: conformation is said to be staggered when groups (attached to front and back carbons) are not aligned in the same axis.
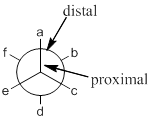
Eclipsed conformation: conformation is said to be eclipsed when groups (attached to front and back carbons) are aligned in the same axis.
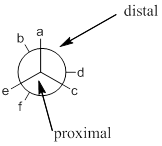
Because of the high torsional strain between atoms of eclipsed conformations, makes them more energized as compared to staggered conformations.
For eclipsed conformations, Torsional strain and steric interactions of atoms are the energizing factors.
For staggered conformations, anti-interactions and gauche interactions of atoms are the energizing factors.
To rank: the given conformations in order of increasing energy.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





