
To draw:
a) The Lewis structures for four resonance forms of linear N5-.
b) Calculate formal charges and identify the structures that contribute the most to the bonding in N5-.
c) By comparing Lewis structures for N5- and N3-, find out the ion in which the nitrogen-nitrogen bonds have the higher average bond order.
Answer to Problem 4.175QA
Solution:
a)
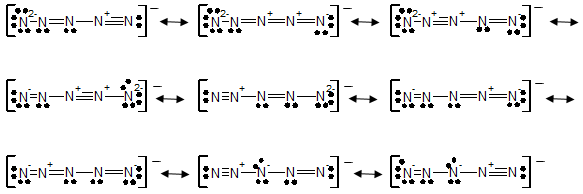
b) The last four structures having the lowest formal charge. These structures contribute the most to the bonding in N5-.
c)
![]()
From these resonance structures we see that each bond is predicted to be of double bond character in N3-. Therefore, in N5- there are two longer nitrogen-nitrogen bonds than in N3-. N3- has the higher average bond order.
Explanation of Solution
The ground state electronic configuration of N is 1s2 2s2 2p3.
| Element | Valence electrons | ||
| Symbol | # of atoms | In one atom | Total |
| N | 5 | 5 | 25 |
| One electron from -1 charge | 1 | ||
| Valence electrons in molecule | 26 | ||
Each N atom is attached with single bond.
N - N - N - N - N
Eight electrons get involved in the bond formation. Now we put remaining electrons as lone pairs on the atoms.
![]()
The octet of terminal N atoms is get completed. The octet of remaining N is not get completed. For that we will convert some lone pairs into bond pairs.

Now we calculate the formal charge on each atom in second structure.
The Lewis structure with formal charge on N atom is as follows.

![]()
Now we draw the resonating structures of this molecule.
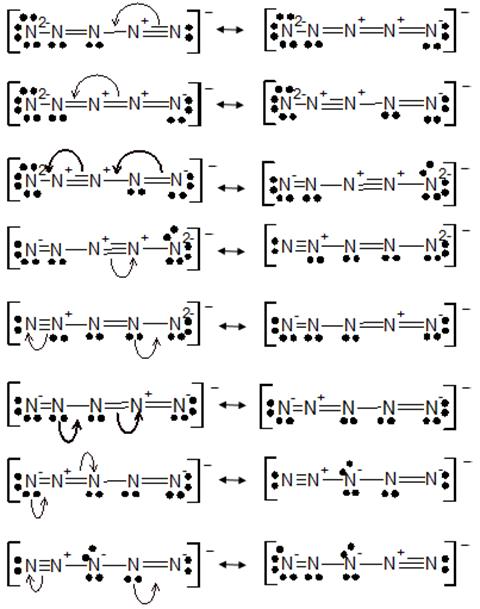
The molecules which having minimum formal charge will contribute the most to the bonding in N5-. Following structures having least formal charge and will contribute the most to the bonding in N5-.
![]()
![]()
![]()
![]()
Let’s we draw the Lewis structure of N3-.
| Element | Valence electrons | ||
| Symbol | # of atoms | In one atom | Total |
| N | 3 | 5 | 15 |
| One electron from -1 charge | 1 | ||
| Valence electrons in molecule | 16 | ||
![]()
From these resonance structures we see that each bond is predicted to be of double bond character. Therefore, in N5- there are two longer nitrogen-nitrogen bonds than in N3-.
Bond order is the number of bonds between atoms. For single bond the bond order is 1. For double bond the bond order is 2. For triple bond the bond order is 3. From the resonance structure of N5- and N3-, we can say that N3- has the higher average bond order.
Conclusion:
From the electronic configuration of atoms in the molecule we can draw Lewis structure of the molecule. From the Lewis structure we can find out formal charge and bond order.
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry: An Atoms-Focused Approach
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





