
To find how formal charges are used to choose between possible molecular structures.
Explanation of Solution
1) Concept :
A Formal charge (FC) is not a real charge to calculate the charge of an individual atom in a molecule or polyatomic ion. It is used to assign the number of electrons to an atom in a structure.
2) Formula:
Using this formula FC on an atom in a molecule or polyatomic ion can be calculated.
3) Calculation:
Let’s take the example of N2O molecule. The various resonance structures possible for N2O molecule are:
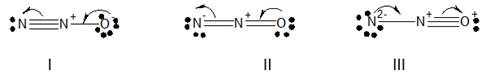
In structure (I),
Therefore, the sum of FC on three atoms = (0) + (+1) + (-1) = 0
In structure (II),
Therefore, the sum of FC on three atoms = (-1) + (+1) + (0) = 0
In structure (III),
Therefore, the sum of FC on three atoms = (-2) + (+1) + (+1) = 0
Now to decide the best or most contributing resonance structures, some rules or criteria have to be followed.
Criteria to choose best molecular structure
a) The best structure shall be one in which each atom has formal charge equal to zero.
b) If this is not possible, then structure having most of the atoms carrying zero or nearly zero charge shall be considered.
c) Negative charge should reside on more electronegative element(s)
Considering these three criteria, it can be concluded that, among the structures I, II and III, structure (I) is best structure to represent N2O molecule.
Structure (III) is least contributing structure and (II) can be considered as the intermediate between (I) and (III).
Conclusion:
To choose the best resonance structure in a molecule or in a polyatomic ion, the above three criteria should be followed.
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry: An Atoms-Focused Approach
- Ph heat heatarrow_forward(12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





