
(a)
Interpretation:
The given structure has to be classified as an aldehyde, a ketone or neither.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
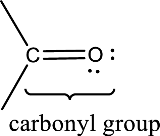
An aldehyde is a carbonyl compound in which the carbonyl carbon atom is bonded to at least one hydrogen atom directly. The other group attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
A ketone is a carbonyl compound in which the carbonyl carbon atom is bonded to two other carbon atoms directly. The groups attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
(b)
Interpretation:
The given structure has to be classified as an aldehyde, a ketone or neither.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
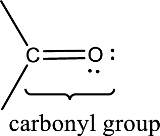
An aldehyde is a carbonyl compound in which the carbonyl carbon atom is bonded to at least one hydrogen atom directly. The other group attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
A ketone is a carbonyl compound in which the carbonyl carbon atom is bonded to two other carbon atoms directly. The groups attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
(c)
Interpretation:
The given structure has to be classified as an aldehyde, a ketone or neither.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
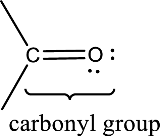
An aldehyde is a carbonyl compound in which the carbonyl carbon atom is bonded to at least one hydrogen atom directly. The other group attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
A ketone is a carbonyl compound in which the carbonyl carbon atom is bonded to two other carbon atoms directly. The groups attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
(d)
Interpretation:
The given structure has to be classified as an aldehyde, a ketone or neither.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
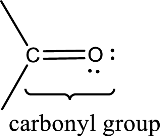
An aldehyde is a carbonyl compound in which the carbonyl carbon atom is bonded to at least one hydrogen atom directly. The other group attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
A ketone is a carbonyl compound in which the carbonyl carbon atom is bonded to two other carbon atoms directly. The groups attached to the carbonyl carbon atom can be alkyl, cycloalkyl, or aryl group.
Trending nowThis is a popular solution!

Chapter 4 Solutions
Organic And Biological Chemistry
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





