
(a)
Interpretation:
The structural formulas of the product formed from hydrolysis of given acetal in an acid solution has drawn.
Concept Introduction:
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Acetals are stable in basic solution. But in acidic solution, they undergo hydrolysis. Hydrolysis is a
Acetal is formed when the formed hemiacetal reacts with further alcohol molecule so that the hydroxyl group in the hemiacetal is converted into alkoxy group. This can be shown as given below,
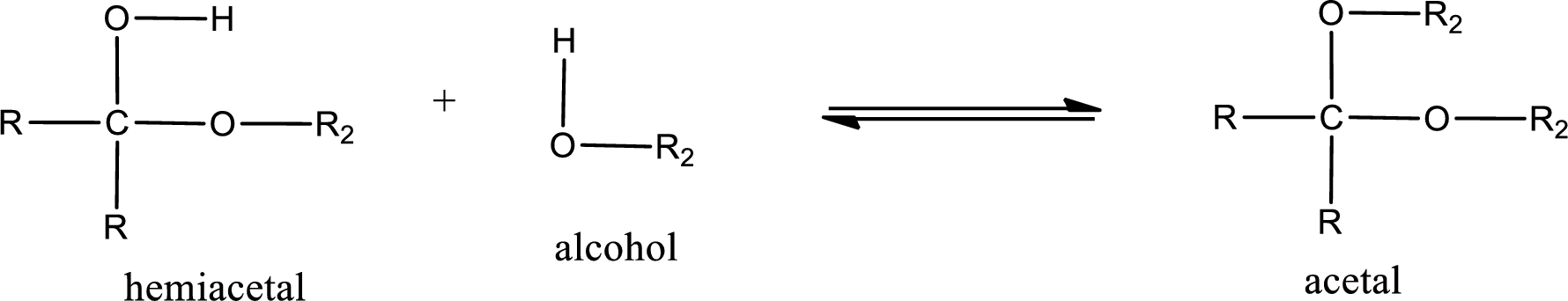
Acetal undergoes hydrolysis in acidic solution to form two alcohol molecules and ketone or aldehyde molecule. The general reaction for hydrolysis of acetal in acid solution,
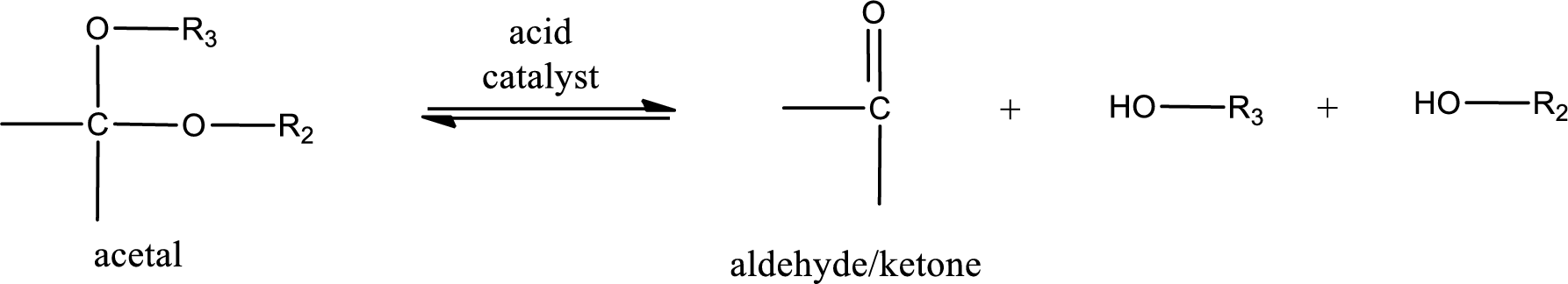
(b)
Interpretation:
The structural formulas of the product formed from hydrolysis of given acetal in an acid solution has drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Acetals are stable in basic solution. But in acidic solution, they undergo hydrolysis. Hydrolysis is a chemical reaction in which the compound splits into two or more fragments when water is added to the compound in presence of acid or base as catalyst. Acetals undergo hydrolysis to give the respective starting materials from which it is formed.
Acetal is formed when the formed hemiacetal reacts with further alcohol molecule so that the hydroxyl group in the hemiacetal is converted into alkoxy group. This can be shown as given below,
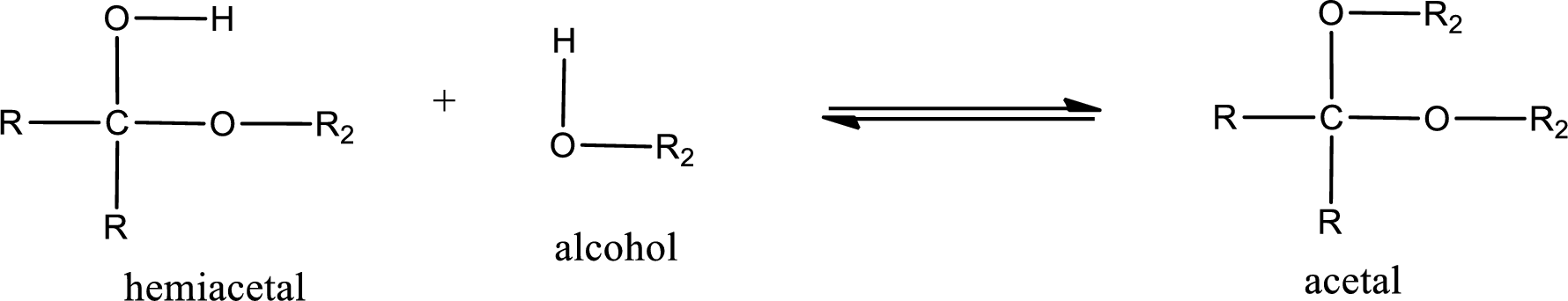
Acetal undergoes hydrolysis in acidic solution to form two alcohol molecules and ketone or aldehyde molecule. The general reaction for hydrolysis of acetal in acid solution,
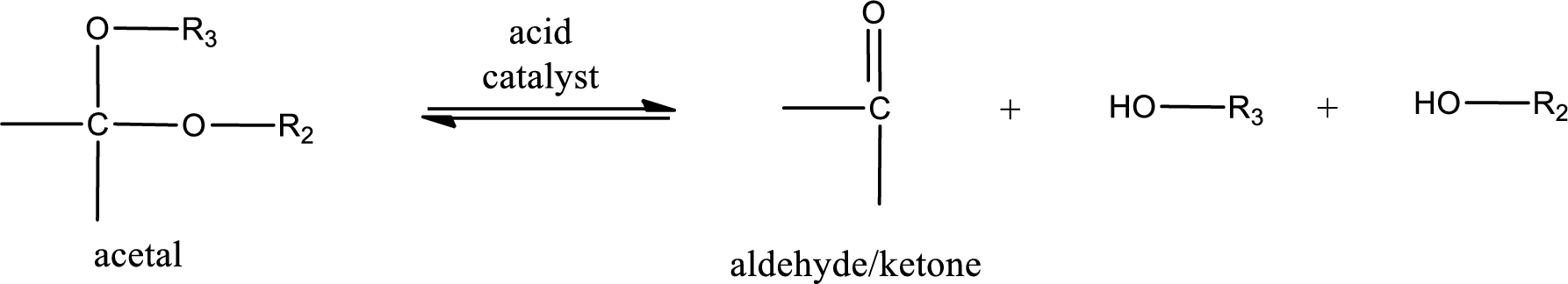
(c)
Interpretation:
The structural formulas of the product formed from hydrolysis of given acetal in an acid solution has drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Acetals are stable in basic solution. But in acidic solution, they undergo hydrolysis. Hydrolysis is a chemical reaction in which the compound splits into two or more fragments when water is added to the compound in presence of acid or base as catalyst. Acetals undergo hydrolysis to give the respective starting materials from which it is formed.
Acetal is formed when the formed hemiacetal reacts with further alcohol molecule so that the hydroxyl group in the hemiacetal is converted into alkoxy group. This can be shown as given below,
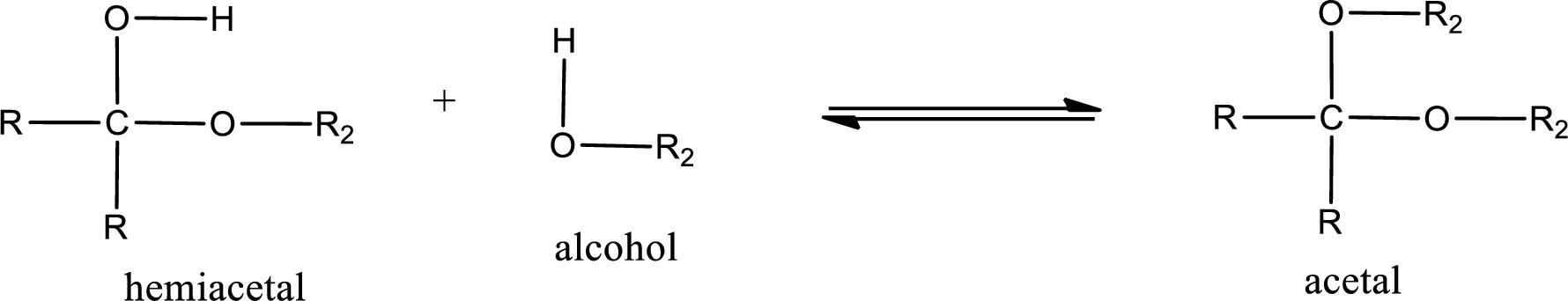
Acetal undergoes hydrolysis in acidic solution to form two alcohol molecules and ketone or aldehyde molecule. The general reaction for hydrolysis of acetal in acid solution,
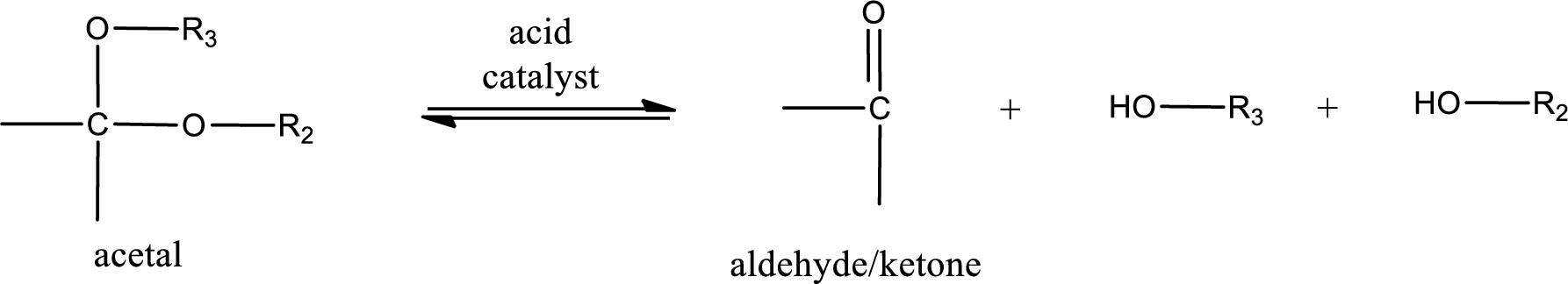
(d)
Interpretation:
The structural formulas of the product formed from hydrolysis of given acetal in an acid solution has drawn.
Concept Introduction:
Aldehydes contain a carbonyl group that is bonded to a hydrogen atom and a carbon atom. Ketones are compounds that contain a carbonyl group bonded to two carbon atoms. Aldehydes and ketones undergo addition reaction across the carbonyl group.
Aldehydes and ketones react with alcohol to form hemiacetal as the product. This reacts with further molecule of aldehyde or ketone to form acetal.
Acetals are stable in basic solution. But in acidic solution, they undergo hydrolysis. Hydrolysis is a chemical reaction in which the compound splits into two or more fragments when water is added to the compound in presence of acid or base as catalyst. Acetals undergo hydrolysis to give the respective starting materials from which it is formed.
Acetal is formed when the formed hemiacetal reacts with further alcohol molecule so that the hydroxyl group in the hemiacetal is converted into alkoxy group. This can be shown as given below,
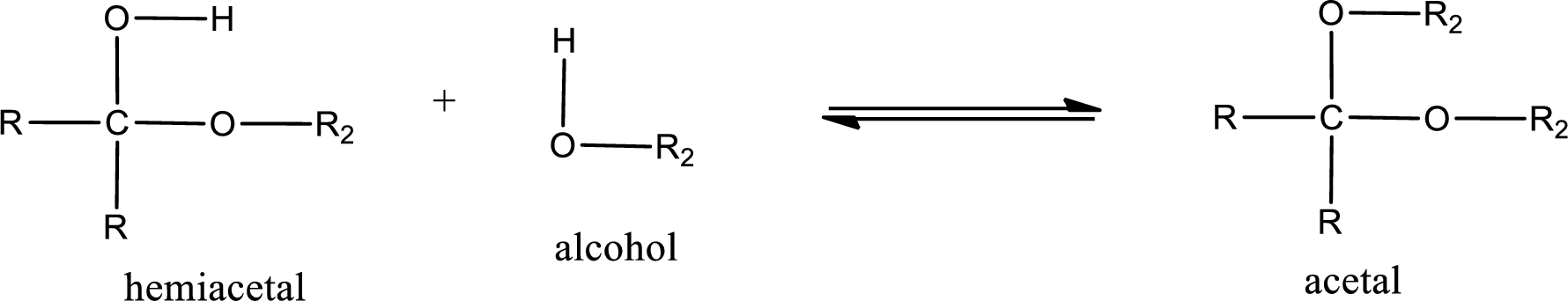
Acetal undergoes hydrolysis in acidic solution to form two alcohol molecules and ketone or aldehyde molecule. The general reaction for hydrolysis of acetal in acid solution,
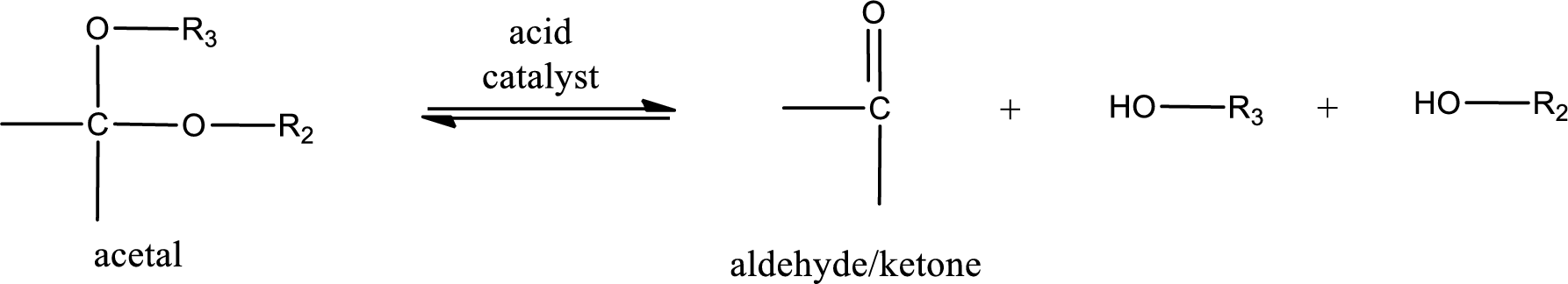
Trending nowThis is a popular solution!

Chapter 4 Solutions
Organic And Biological Chemistry
- enumerate the properties of alcohols contributing to their reactivity with an oxidizing reagent?arrow_forwardExplain the characteristic reaction of aldehydes and ketones ?arrow_forward4. Write a general equation representing the preparation of an alcohol by hydrogenation of an aldehyde or a ketone.arrow_forward
- Explain the process associated with the acylation of benzene?arrow_forwardGive the structure of an alcohol that could be used to prepare each of the following compounds: a. b. c.arrow_forwardDraw structural formulas for the following entities. a. Propanoate ion b. Sodium propanoate c. Acetate ion d. Sodium acetatearrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





