
Concept explainers
Give the IUPAC name for each compound.
a.
b.
c.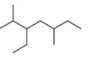
d.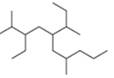
(a)
Interpretation: The IUPAC name of the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.10P
The IUPAC name of the given compound is
Explanation of Solution
The given compound is
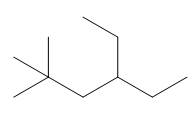
Figure 1
One should follow the given four steps to give the IUPAC name of a compound.
The first step is naming of longest parent chain.
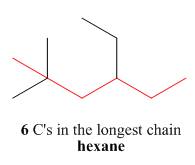
Figure 2
The second step is numbering of chain.
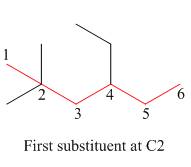
Figure 3
The third step is naming and numbering of substituents.
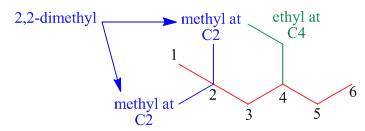
Figure 4
The fourth step is combining of all parts.
Thus, the IUPAC name of the given compound is
The IUPAC name of the given compound is
(b)
Interpretation: The IUPAC name of the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.10P
The IUPAC name of the given compound is
Explanation of Solution
The given compound is
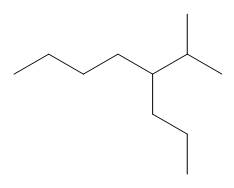
Figure 9
One should follow the given four steps to give the IUPAC name of a compound.
The first step is naming of longest parent chain.
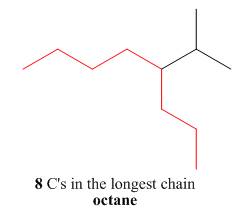
Figure 10
The second step is numbering of chain.
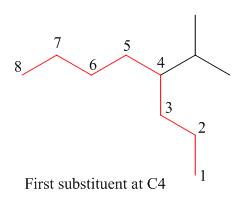
Figure 11
The third step is naming and numbering of substituents.
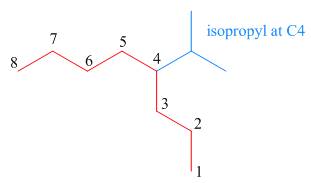
Figure 12
The fourth step is combining of all parts.
Thus, the IUPAC name of the given compound is
The IUPAC name of the given compound is
(c)
Interpretation: The IUPAC name of the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.10P
The IUPAC name of the given compound is
Explanation of Solution
The given compound is,
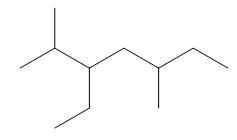
Figure 5
One should follow the given four steps to give the IUPAC name of a compound.
The first step is naming of longest parent chain.
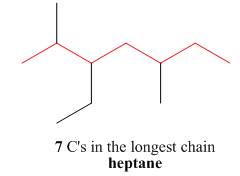
Figure 6
The second step is numbering of chain.
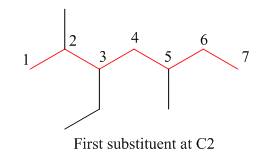
Figure 7
The third step is naming and numbering of substituents.
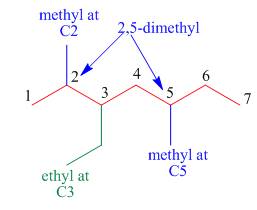
Figure 8
The fourth step is combining of all parts.
Thus, the IUPAC name of the given compound is
The IUPAC name of the given compound is
(d)
Interpretation: The IUPAC name of the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.10P
The IUPAC name of the given compound is
Explanation of Solution
The given compound is,
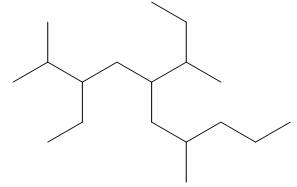
Figure 13
One should follow the given four steps to give the IUPAC name of a compound.
The first step is naming of longest parent chain.
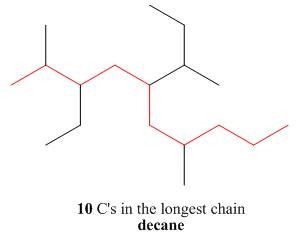
Figure 14
The second step is numbering of chain.
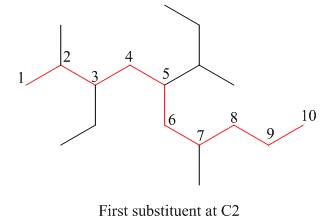
Figure 15
The third step is naming and numbering of substituents.
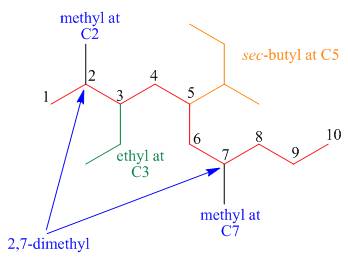
Figure 16
The fourth step is combining of all parts.
Thus, the IUPAC name of the given compound is
The IUPAC name of the given compound is
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry
- Name each compound in which the benzene ring is best treated as a substituent. CH3 a. CH3-CH-CH,-CH-CH,-CH–CH,-CH; CH,-CH3 b. CH,-CH-CH=CH-CH,-CH,–CH,-CH, c. CH3-C=C-CH-CH-CH-CH2-CH3 CH3 CH3arrow_forwardB. Give the IUPAC name for each compound. F CH3 ÇI 1. CH;CH2CHCH2CH2CHCH3 5. CH,CH3 F 2. CI 6. OH CI NO, 7. NO, NO, Br 8. Br 3.arrow_forwardDraw the products of combustion of each alkane. a. CH;CH,CH,CH2CH(CH3)2 b.arrow_forward
- 7. Name the following phenols. b) OH c) d) a) OH Br- HO CI Br 8. Name the following ethers. b) a) CHy-0-CH-CHy CH-0-CH3 c) d) Cゅ-C-oarrow_forwardWhich alkyl halide has the highest boiling point? A. CH3BrB. CH3FC. CH3ClD. CH3larrow_forwardGive a systematic (IUPAC) name for each diol.(a) CH3CH(OH)(CH2)4CH(OH)C(CH3)3arrow_forward
- Draw the structure of a molecule that fi ts each description: a. a 2 ° alcohol of molecular formula C 6H 12O b. a cyclic ether with molecular formula C 5H 10O c. a 1 ° alkyl halide with molecular formula C 5H 11Clarrow_forwardcdn.fbsbx.com I. What products are formed when each alcohol is oxidized with K,Cr,0,? a. CH,CH,CH,CH,CH,OH OH OH H3C CH3 b. C. II, Give the structure corresponding to each IUPAC name:arrow_forwardName each alkene: a. CH3CH=CHCH2CH2CH3 b. CH3CH=CH2 c. CH2CH3 l CH2=CHCH2CHCH3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





