
Concept explainers
(a)
Interpretation:
The name of given binary compound is to be written.
Concept introduction:
To name a compound, certain rules are followed. The given compounds are binary compounds.
Binary compounds are of three types:
- Type-I: Compound in which a metal forms ionic bond with a non-metal and the metal can form only one type of ions.
- Type-II: Compound in which a metal forms ionic bond with a non-metal and the metal can form more than one type of ions.
- Type-III: Compound in which a non-metal forms bond with a non-metal.
(a)
Answer to Problem 12A
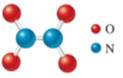
Dinitrogentetraoxide
Explanation of Solution
The structure shows that formula is
Rules for naming Type-III compounds:
- It is name by writing the name of first element as same as element.
- Second element in anionic form and prefixes are used to denote the number of atoms. Prefix mono is not used for 1 element.
The binary compound
(b)
Interpretation:
The name of given binary compound is to be written.

Concept introduction:
To name a compound, certain rules are followed. The given compounds are binary compounds.
Binary compounds are of three types:
- Type-I: Compound in which a metal forms ionic bond with a non-metal and the metal can form only one type of ions.
- Type-II: Compound in which a metal forms ionic bond with a non-metal and the metal can form more than one type of ions.
- Type-III: Compound in which a non-metal forms bond with a non-metal.
(b)
Answer to Problem 12A
Iodine trichloride
Explanation of Solution
The structure shows that formula is
Rules for naming Type-III compounds:
- It is name by writing the name of first element as same as element.
- Second element in anionic form and prefixes are used to denote the number of atoms. Prefix mono is not used for 1element.
The binary compound
(c)
Interpretation:
The name of given binary compound SO2is to be written.
Concept introduction:
To name a compound, certain rules are followed. The given compounds are binary compounds.
Binary compounds are of three types:
- Type-I: Compound in which a metal forms ionic bond with a non-metal and the metal can form only one type of ions.
- Type-II: Compound in which a metal forms ionic bond with a non-metal and the metal can form more than one type of ions.
- Type-III: Compound in which a non-metal forms bond with a non-metal.
(c)
Answer to Problem 12A
Sulphur dioxide
Explanation of Solution
The binary compound
Rules for naming Type-III compounds:
- It is name by writing the name of first element as same as element.
- Second element in anionic form and prefixes are used to denote the number of atoms. Prefix mono is not used for 1 element.
herefore, it is named as Sulphur dioxide to indicate two oxygen atoms.
(d)
Interpretation:
The name of given binary compound P2S3 is to be written.
Concept introduction:
To name a compound, certain rules are followed. The given compounds are binary compounds.
Binary compounds are of three types:
- Type-I: Compound in which a metal forms ionic bond with a non-metal and the metal can form only one type of ions.
- Type-II: Compound in which a metal forms ionic bond with a non-metal and the metal can form more than one type of ions.
- Type-III: Compound in which a non-metal forms bond with a non-metal.
(d)
Answer to Problem 12A
Diphosphoruspentasulphide
Explanation of Solution
The binary compound
Rules for naming Type-III compounds:
- It is name by writing the name of first element as same as element.
- Second element in anionic form and prefixes are used to denote the number of atoms. Prefix mono is not used for first element.
herefore, it is named as Diphosphoruspentasulphide to indicate two phosphorus atoms and five sulphur atoms.
Chapter 4 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





