
Concept explainers
It is observed that as temperature is increased, most protein molecules go from their defined, folded state into a random-coil, denatured state that exposes more hydrophobic surface area than is exposed in the folded state.
a. Given what you have learned so about
b. Sometimes, however, proteins as temperature is decreased. How might this be explained? (Hint: Consider Problem 8)
7. Assume that some protein molecule, in its folded native state, has one favored conformation. But when it is denatured, it becomes a “random coil,” with many possible conformations.
a. If we only consider the entropy for the protein, what must be the sign of
b. How will the contribution of
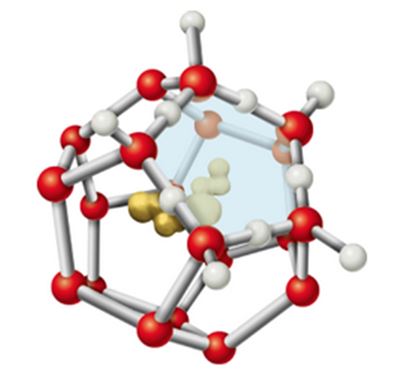
8. When a hydrophobic substance like a hydrocarbon is dissolved in water, a clathrate cage of ordered water molecules is formed about it (see Figure 2.14). If we consider only the effects on water, what do you expect the sign of

Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
- SDS-PAGE gels are used to study changes in protein primary structure. Which of the following statements about SDS-PAGE gels is correct? A. The negatively charged proteins will move towards the negatively charged electrode in the SDS-Page chamber B. A detergent is needed to disrupt protein secondary, tertiary, and quaternary structure before a gel can be run C. No "stains" or other treatments are needed to "see" the protein bands on a gel D. Long polypeptides move a further distance from the loading well in a SDS-PAGE gel than short polypeptidesarrow_forwardWhich of the following statements is incorrect? A.- the structure and charge of the proteins have direct effects on the rate of its migration through the gel matrix include b.- SDS is a type of a buffer with strong protein-denaturing effect and binds to the protein backbone at a constant molar ratio c.- SDS and polyacrylamide gel largely eliminates the influence of the structure and charge, and proteins are separated solely based on polypeptide chain length. d.When proteins are separated by electrophoresis through a gel matrix, smaller proteins appears at the lower portion of the gelarrow_forwardA sample mixture consists of three proteins with the following properties: Protein A MW (kDa) 200 Amino acid composition 40% nonpolar, 60% polar B 45 20% nonpolar, 80% polar C 98 85% nonpolar, 15% polar IpH 9 3 5 1. If the mixture is subjected to ammonium sulfate precipitation, which protein will precipitate out first? [Select] 2. If the mixture is subjected to isoelectric focusing, which protein will stop moving nearest to the positive electrode? [Select] 3. If the mixture is subjected to cation-exchange chromatography using a buffer at pH 7, which protein will bind to the resin? [Select] 4. If the mixture is subjected to SDS-PAGE, which protein will be at the bottommost portion of the gel? [Select] * Previous Nexarrow_forward
- Protein X binds to molecule A. When [A] = 24.5 µmol L-1 at equilibrium, half of X is bound to A and half is not. Calculate the Kd. (Multiply your answer by 1,000,000 before entering. Hint: remember to pay attention to units, and to remember how Kd relates to being half bound.)arrow_forwardThe addition of ethanol, CH3CHOH, t an aqueous solution lowers the surface tension of the solution. Predict whether adding ethanol to an aqueous protein solution will tend to stabilize or unfold the protein. Briefly explain.arrow_forwardConsider five proteins with the properties shown in the following table. Answer the questions below about these proteins and justify your answers. Protein Subunit mass (Da) | Native mass (Da) 10,000 40,000 120,000 A 15,000 C 20,000 20,000 25,000 75,000 E 30,000 60,000 a) Which protein would migrate the slowest in an SDS-PAGE? Justify your results. b) Which protein would migrate the fast in a Native-PAGE? Justify your results.arrow_forward
- Consider the following situation; Fresh pineapple contains the enzyme bromelain that hydrolyzes peptide bonds in proteins. a. The directions in making a gelatin (protein) dessert say not to add fresh pineapple. However, canned pineapple where pineapple is heated to high temperatures can be added. Why? b. Fresh pineapple is used in a marinade to tenderize tough meat. Why? c. What structural level of a protein does the bromelain enzyme destroy?arrow_forwardWhich of the following statements are correct about the role of chaperones in protein folding (select all that appy)? A. Chaperones accelerate the rate of protein folding B. Presence of chaperones contradicts dogma that structure of protein is only determined by amino acid sequence C. Chapreone promotion of native folding is an ATP-dependent process D. Chaperones work by stabilizing hydrophobic patches on proteins E. A cell line with the GroEL and GroES deleted would accumulate more unfolded proteinarrow_forward. Assume that some protein molecule, in its folded native state, has one favored conformation. But, when it is denatured, it becomes a "random coil," with many possible conformations. (a) What must be the sign of AS for the change: native → denatured? (b) How will the contribution of AS for native → denatured affect the favorability of the process? What apparent requirement does this impose on AH if proteins are to be stable structures?arrow_forward
- Assume that some protein molecule, in its folded native state, has onefavored conformation. But when it is denatured, it becomes a “random coil,” with many possible conformations.(a) If we only consider the change in entropy for the protein, what mustbe the sign of ΔS for the change: native → denatured? (Note: As suggestedin the next problem, this does not include solvent effects, which also make significant contributions to ΔS.)(b) How will the contribution of ΔS for native → denatured affect thefavorability of the process? What apparent requirement does this imposeon ΔH if proteins are to be stable structures?arrow_forward11. Below is a folding energy funnel describing folding energy landscape of a protein. The width of the funnel indicates the entropy of the protein, and the height corresponds to the free energy. A) If A is the native fold structure, which state is a molten globule? How does this state differ from A in term of structures. B) Does this protein have multiple folding pathways or just one? C) which state has the lowest free energy? D) According to the width of the funnel, the native state B of the protein has the lowest entropy. If the protein fold A spontaneously to this state, does it violate the 2nd law of thermodynamics? Why or why not? (Hint: in the folding funnel, only the entropy of the protein alone is considered). E) Does the native state also have the lowest enthalpy. What makes the enthalpy decrease as the protein folds? 12. List four methods by which a protein can be denatured and briefly describe how these methods act to disrupt protein structure.arrow_forwardIn the protein denaturation experiment, which of the following can be a consequence of the air bubbles in the viscometry run for an aqueous solution of protein with a denaturant?a. Increased t0b. Decreased t0c. Increased nspd. Decreased nspAll of the statements about protein denaturation are true EXCEPT:a. The viscosity of linear proteins is greater than that of the globular proteins.b. Only the BME can disrupt a covalent bond while the other denaturants can just disrupt non-covalent bonds.c. Protein renaturation is possible but in some cases protein denaturation proceeds to protein degradation. d. Protein denaturation generally disrupts tertiary or quaternary structures onlyarrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





