
a)
Interpretation : The approximate boiling points for the C2, C4, C6, and C8
Concept Introduction : A measure of an object's heat or cold is its temperature. The temperature of an object determines how heat is transferred.
a)
Answer to Problem 92A
The approximate boiling points for the C2, C4, C6, and C8 alkanes are
Explanation of Solution
The graph is plotted between the number of carbon atoms and the boiling points.
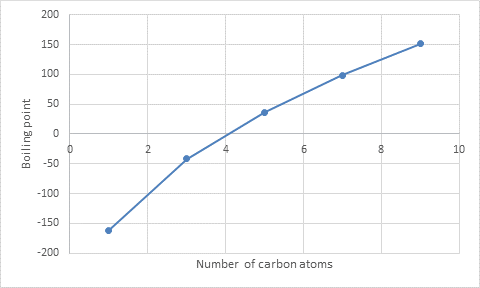
With the help of a graph, the boiling point of C2, C4, C6, and C8 alkanes are obtained.
The approximate boiling point of C2 alkane is
The approximate boiling point of C4alkane is
The approximate boiling point of C6 alkane is
The approximate boiling point of C8alkane is
b)
Interpretation : The nine alkanes that are gases at room temperature are to be identified.
Concept Introduction : A measure of an object's heat or cold is its temperature. The temperature of an object determines how heat is transferred.
b)
Answer to Problem 92A
The alkanes that are gases at room temperature are C1, C2, C3, and C4 alkanes.
Explanation of Solution
The alkanes beyond their boiling point become gaseous.
The alkanes are liquid when it is below their boiling point.
From the graph, the alkanes that are gases at room temperature can be identified.
When the boiling point of alkanes is less than the room temperature, those alkanes become gases at that room temperature.
The alkanes such as C1, C2, C3, and C4 alkanes have less boiling point than room temperature so they become gaseous.
c)
Interpretation : The nine alkanes that are liquid at 350 K are to be identified.
Concept Introduction : A measure of an object's heat or cold is its temperature. The temperature of an object determines how heat is transferred.
c)
Answer to Problem 92A
The alkanes that are liquid at 350 K are C7, C8, and C9 alkanes.
Explanation of Solution
The alkanes beyond their boiling point become gaseous.
The alkanes are liquid when it is below their boiling point.
From the graph, the alkanes that are liquid at 350 K can be identified.
The temperature is 350 K means
When the boiling point of alkanes is higher than 350 K, those alkanes are liquid at that temperature.
The alkanes such as C7, C8, and C9 alkanes have high boiling point than 350 K so they are liquid at that temperature.
d)
Interpretation : The approximate increase in boiling point per additional carbon atom in these alkanes is to be identified.
Concept Introduction : A measure of an object's heat or cold is its temperature. The temperature of an object determines how heat is transferred.
d)
Answer to Problem 92A
The approximate increase in boiling point per additional carbon atom in these alkanes is
Explanation of Solution
With the help of the graph, the approximate increase in boiling point is calculated.
There is approximately
Chapter 3 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- Please correct answer and don't used hand raitingarrow_forward14. Draw all of the products expected for the following reaction. Circle the products expected to predominate when the reaction is heated to 40 °C. EXPLAIN your choice. (12 points) HBr ? Br -11arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardDraw and show the full mechanism of how the molecule ((1E, 3E, 5E)-1-methoxyhepta-1,3,5-triene) is built using substitution and elimination reactions. You can start with an alkane of any carbon length with any number of leaving groups attached or with a alkoxide of any carbon length (conjugate base of an alcohol). Show each step and and explanation for each reaction. Also include why the reagents and solvents were picked and what other products can be expected.arrow_forwardDon't USE AIarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





