
Interpretation : The relationship between Fahrenheit and Celsius temperature is to be derived.
Concept Introduction : A measure of an object's heat or cold is its temperature.
The temperature of an object determines how heat is transferred.
Answer to Problem 81A
The equation for the relationship between Fahrenheit and Celsius temperature is given as:
Explanation of Solution
Given information:
| Example | ||
| Melting point of selenium | ||
| Boiling point of water | ||
| Normal body temperature | ||
| Freezing point of water | ||
| Boiling point of chlorine |
A measure of an object's heat or cold is its temperature.
The temperature of an object determines how heat is transferred.
With the help of the Celsius and Fahrenheit values, a graph is drawn by plotting Celsius in the x-axis and Fahrenheit in the y-axis.
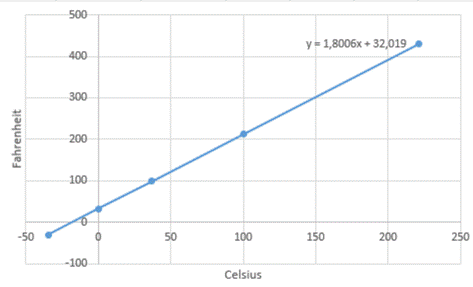
From the graph, it is clear that the relation between Celsius and Fahrenheit gives a linear equation.
The equation is expressed as
To find the equation for Fahrenheit, the following equation is used.
From the table, the values are obtained.
To calculate the equation for Fahrenheit, substitute the values in the above equation.
The equation for the relationship between Fahrenheit and Celsius temperature is given as
Chapter 3 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





