
(a)
Interpretation:
The element that is highlighted in red in the periodic table is present in s area or p area has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
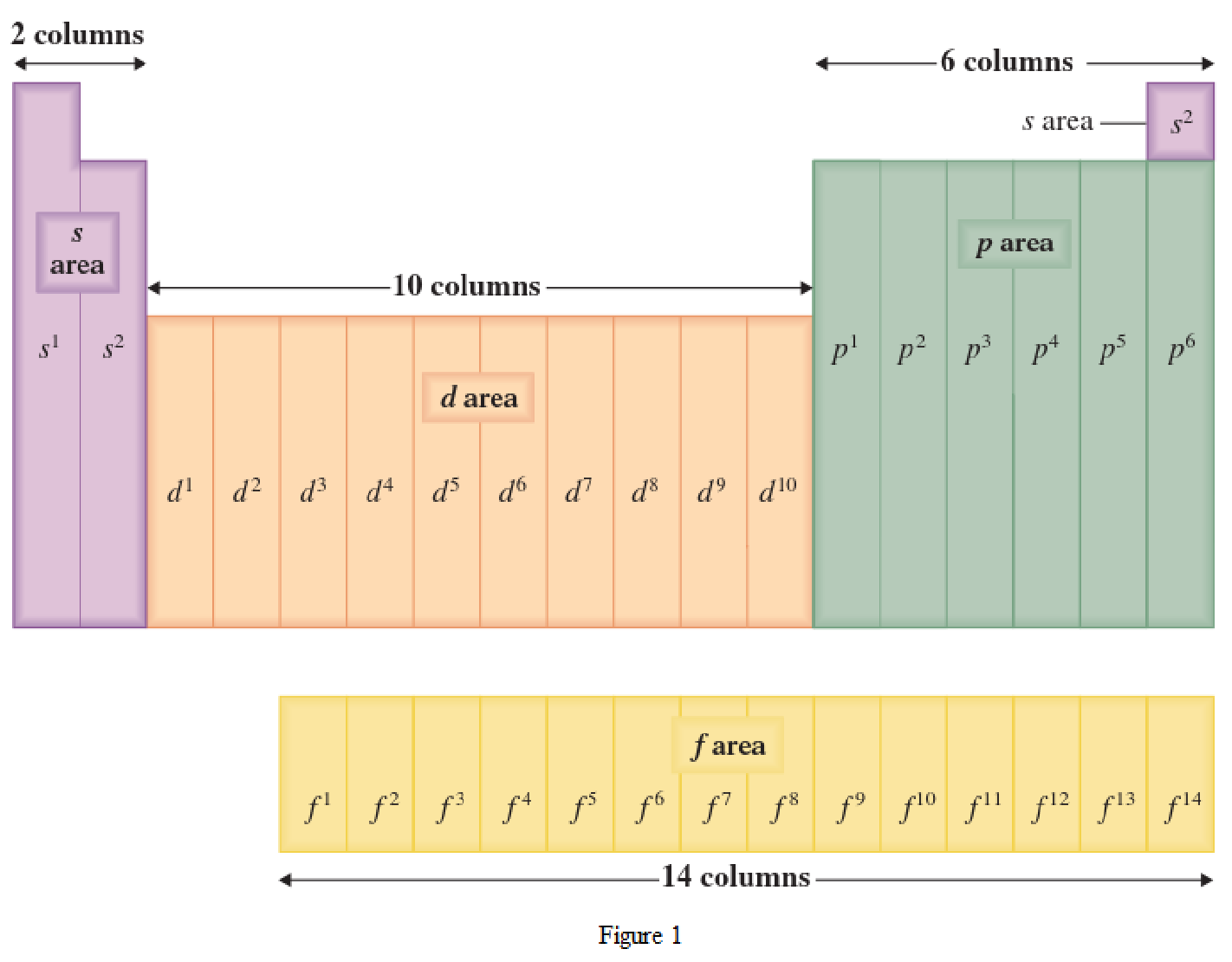
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(b)
Interpretation:
The element that is highlighted in green in the periodic table is present in d area or p area has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
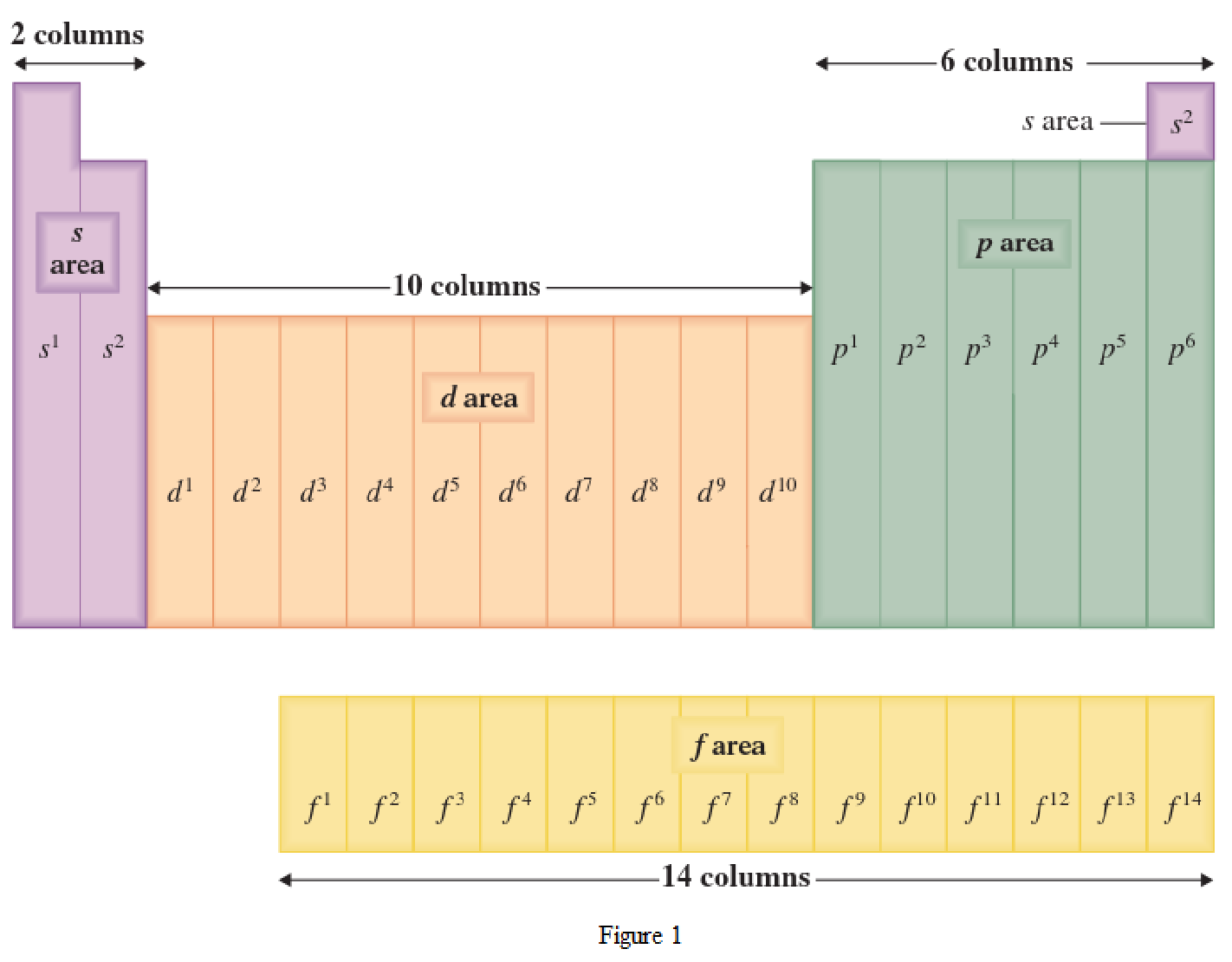
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(c)
Interpretation:
The element that is highlighted in yellow in the periodic table is a p2 or a p4 element has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
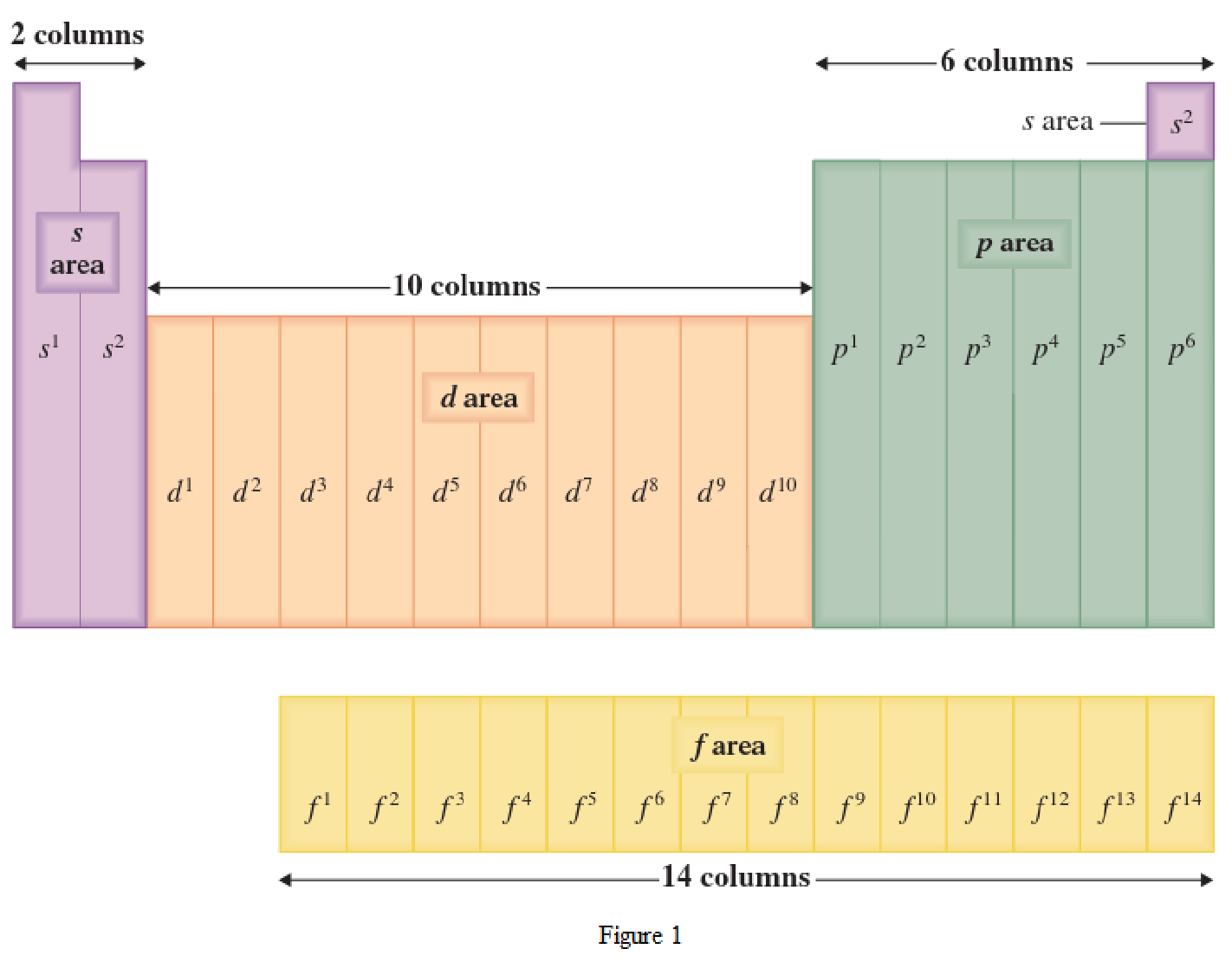
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(d)
Interpretation:
The element that is highlighted in blue in the periodic table is a d2 or a s2 element has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
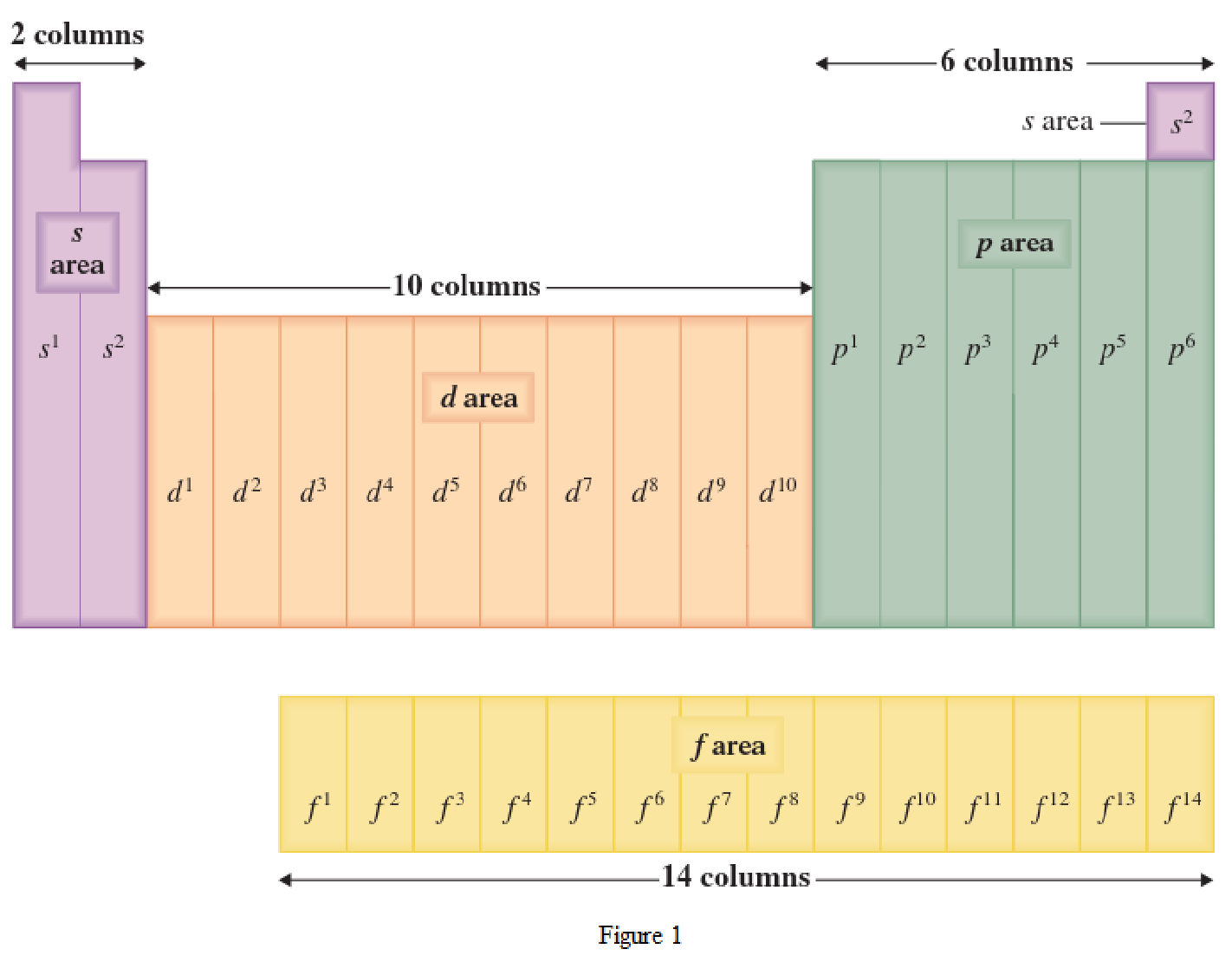
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
Trending nowThis is a popular solution!

Chapter 3 Solutions
General, Organic, and Biological Chemistry
- please helparrow_forwardPredict the products of the following reactions. Draw mechanism arrows for each step for a, b, and c. a.) HBr b.) HI H₂O H2SO4 d.) C12 HO H2SO4 1.) BH3 2.) H2O2, NaOHarrow_forwardK for the following reaction is 0.11 at constant temperature. If the equilibrium concentration of HCl is 0.5 M, what is the equilibrium concentration of NH3. NH4CI(s) ⇌ NH3(g) + HCI(g)arrow_forward
- please help by Draw the following structures (Lewis or line-angle drawing).arrow_forwardplease helparrow_forwardConsider the reaction: 2 A (aq) ⇌ B(aq) Given the following KC values and starting with the initial concentration of A = 4.00 M, complete ICE diagram(s)and find the equilibrium concentrations for A and B.A) KC = 4.00B) KC = 200C) KC = 8.00 x10-3arrow_forward
- 5) Consider the reaction: Cl2 (g) + F2 (g) ⟷ 2 ClF (g) KP=? The partial pressure of 203 kPa for Cl2 and a partial pressure of 405 kPa for F2. Upon reaching equilibrium, thepartial pressure of ClF is 180 kPa. Calculate the equilibrium concentrations and then find the value for KP.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception tothe general ionization energy (IE) trend. For the two elements involved, answer the followingquestions. Be sure to cite sources for all physical data that you use.a. (2 pts) Identify the two elements and write their electronic configurations.b. (2 pts) Based on their configurations, propose a reason for the IE trend exception.c. (5 pts) Calculate effective nuclear charges for the last electron in each element and theAllred-Rochow electronegativity values for the two elements. Can any of these valuesexplain the IE trend exception? Explain how (not) – include a description of how IErelates to electronegativity.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





