
(a)
Interpretation:
The element that is highlighted in yellow in the periodic table is present in p area or d area has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
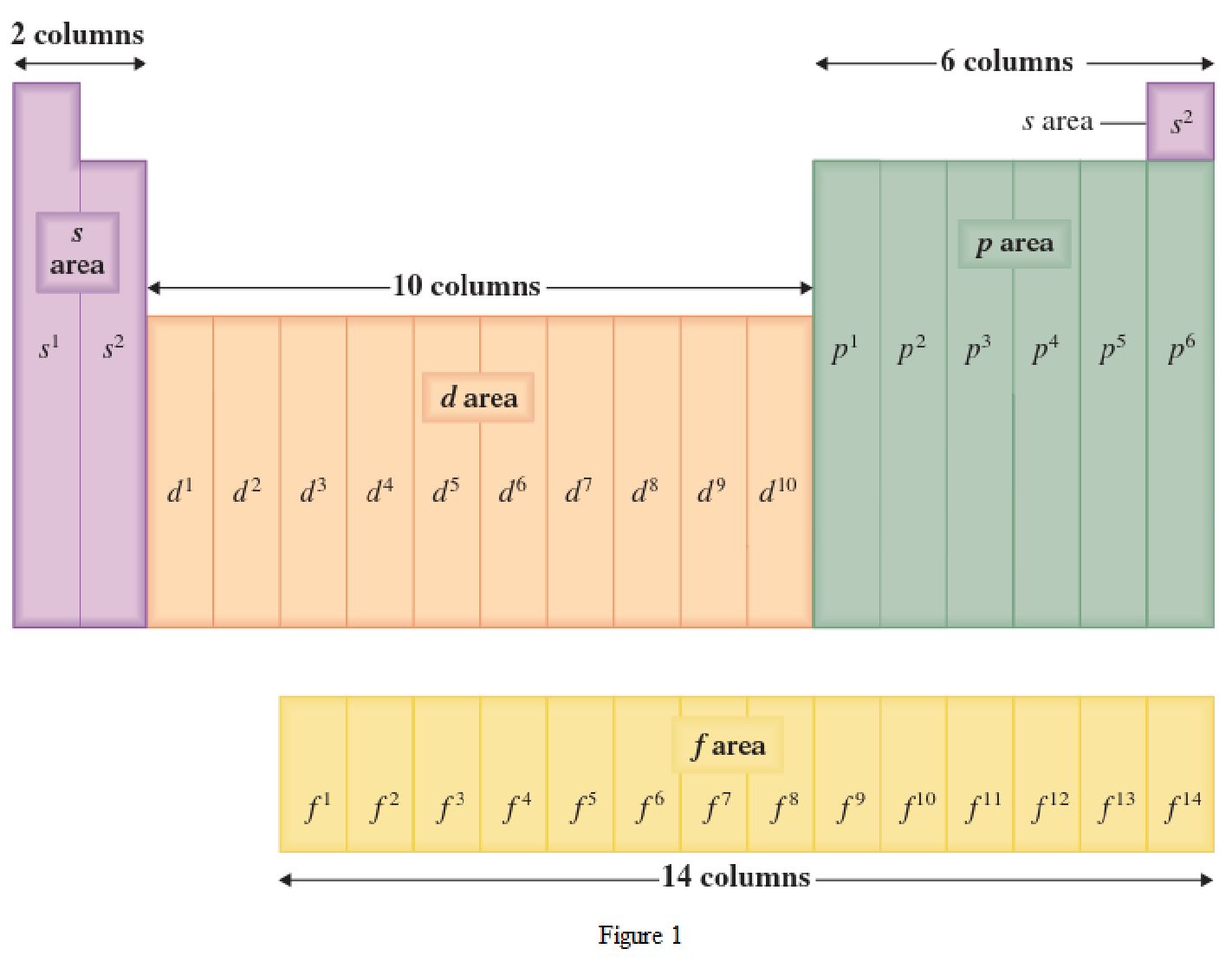
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(b)
Interpretation:
The element that is highlighted in blue in the periodic table is present in s area or d area has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
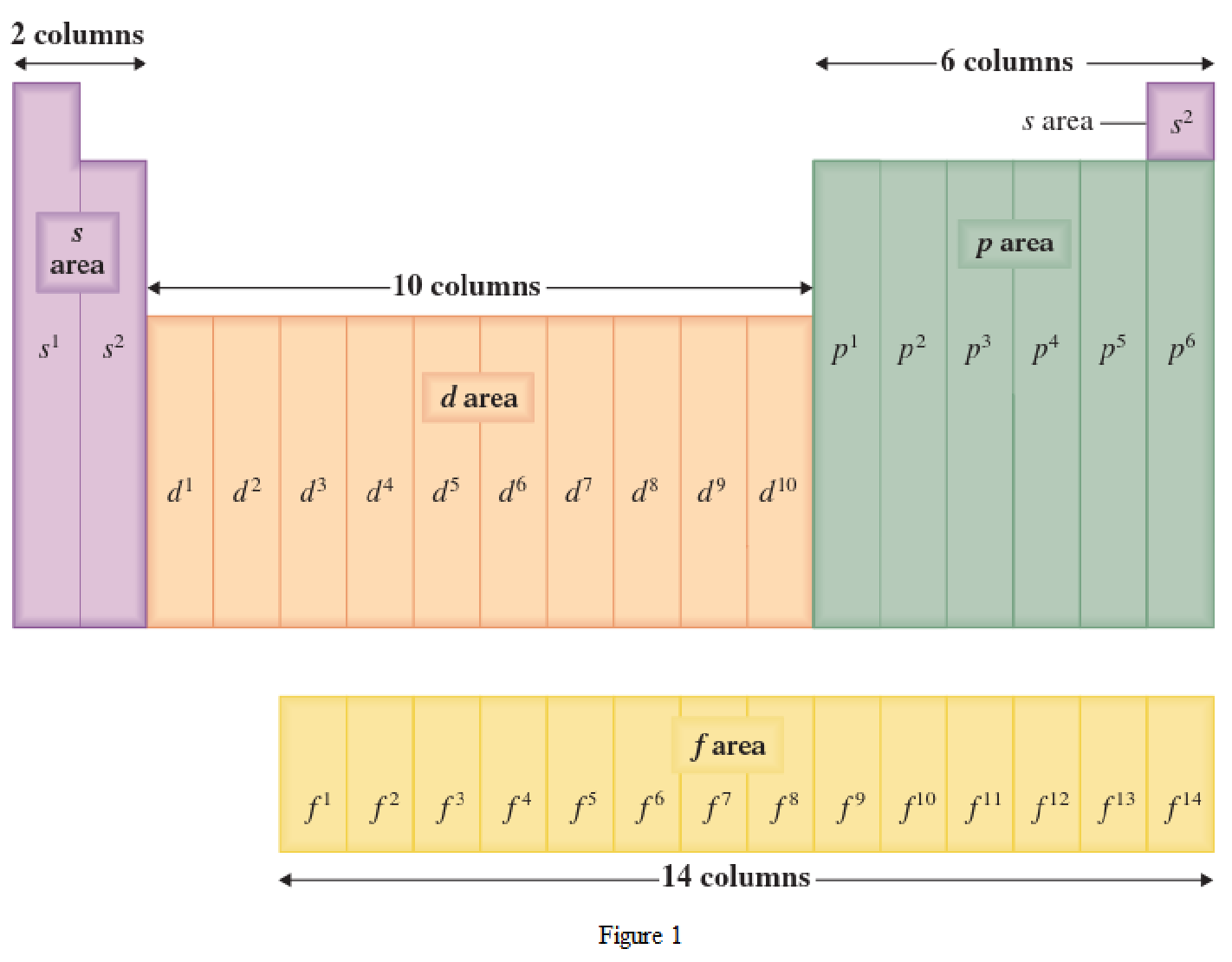
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(c)
Interpretation:
The element that is highlighted in red in the periodic table is a s2 or a d2 element has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
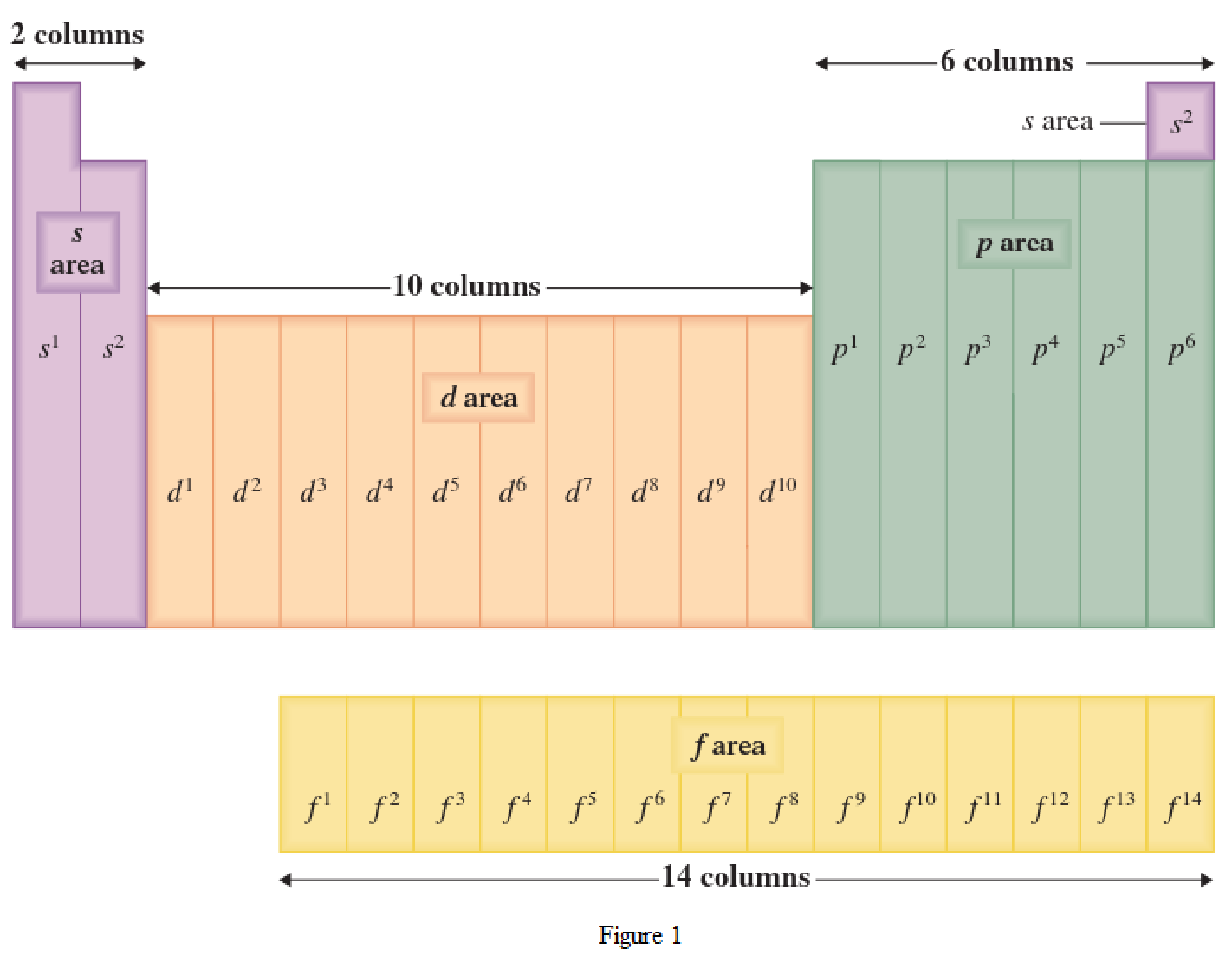
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
(d)
Interpretation:
The element that is highlighted in green in the periodic table is a d6 or a d8 element has to be determined.
Concept Introduction:
Periodic law states that if the elements are arranged in increasing order of atomic number, then the elements with similar chemical properties occur at regular intervals or periodic intervals. The elements are arranged in a periodic table in which the arrangement was based on the atomic number of the elements and the elements that have similar chemical properties are positioned in vertical columns.
Location of an element in a periodic table can be given by the period number and the group number. The horizontal row in a periodic table where the elements are present is known as Period. The vertical column in a periodic table where the elements are present is known as Group.
Chemical properties of the elements repeat themselves at regular intervals because of the electronic configuration. The elements that are present in a Group have similar chemical properties. This is because the outer-shell electronic configuration will be the same.
The periodic table has all the elements that can be distinguished based on the outer-shell electron. If the outer-shell electron is present in s subshell, then the elements are present in s area of periodic table. If the outer-shell electron is present in p subshell, then the elements are present in p area of periodic table. If the outer-shell electron is present in d subshell, then the elements are present in d area of periodic table. If the outer-shell electron is present in f subshell, then the elements are present in f area of periodic table.
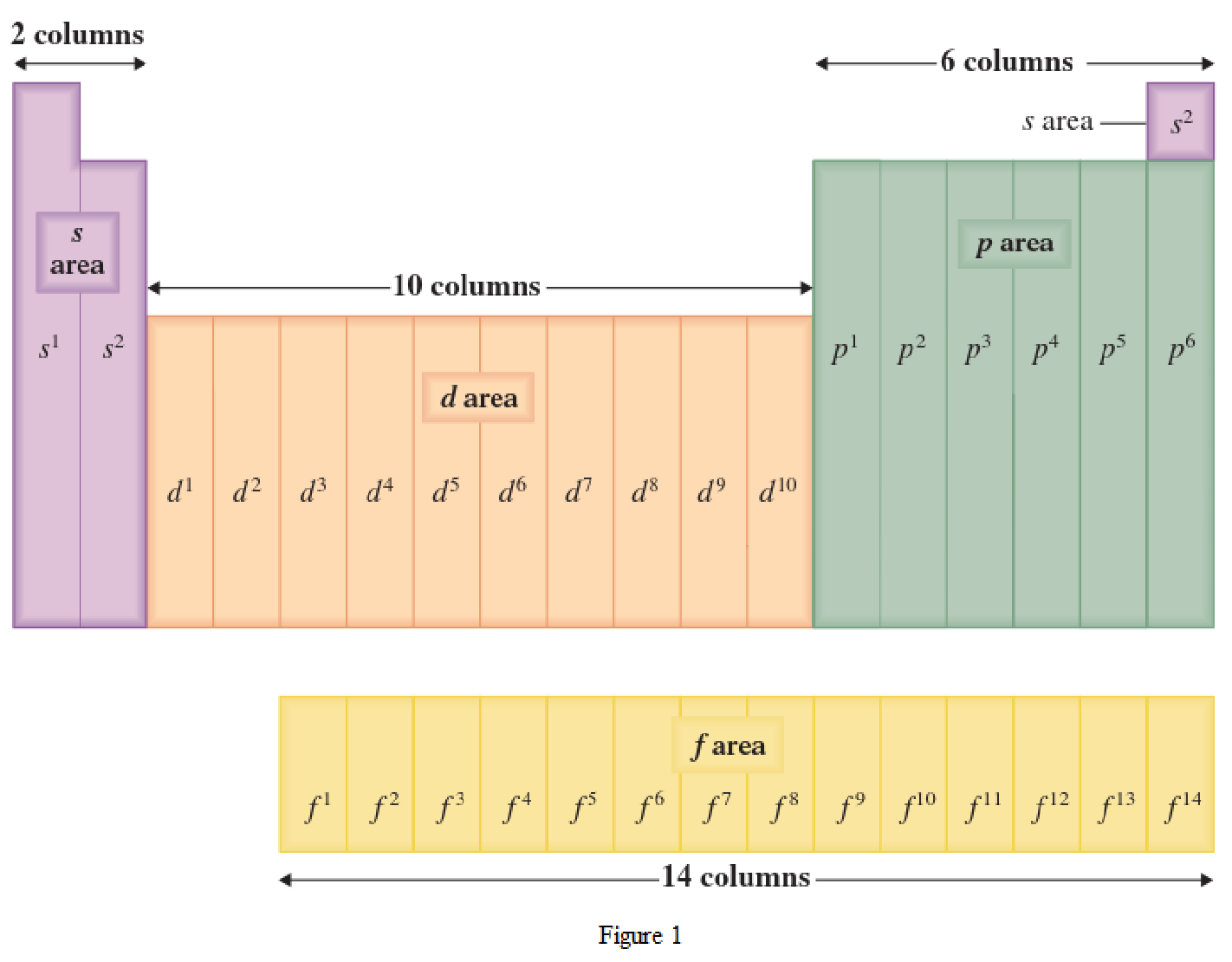
Distinguishing electron is the one that is the last electron added to the electronic configuration of an element when the electron subshells are filled in the order of increasing energy. This distinguishing electron determines the area of the element in the periodic table. This is because this only causes the element electronic configuration to differ from other elements.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
General, Organic, and Biological Chemistry
- Linuron, a derivative of urea, is used as an herbicide. Linuron serum levels were measured in 4Kg rabbits following a bolus IV injection of 10mg/kg. Time (minutes) Serum Linuron Levels (ug/ml) following IV dose 10 15.48 20 8.60 30 5.90 45 3.78 60 2.42 90 1.49 120 0.93 180 0.60 240 0.41 300 0.29 360 0.22 Analyze this data and perform the necessary calculations to determine the following pharmacokinetic parameters from the IV data: (5 points per parameter, 24 parameters/variables ■ 120 points possible). You do NOT need to submit graphs or data tables. Give the terminal regression line equation and R or R² value: Give the x axis (name and units, if any) of the terminal line: Give the y axis (name and units, if any) of the terminal line: Give the residual regression line equation and R or R² value: Give the x axis (name and units, if any) of the residual line: Give the y axis (name and units, if any) of the residual line:arrow_forward3. In the tomato, red fruit (O+) is dominant over orange fruit (0), and yellow flowers (W+) are dominant over white flowers (w). A cross was made between true-breeding plants with red fruit and yellow flowers, and plants with orange fruit and white flowers. The F₁ plants were then crossed to plants with orange fruit and white flowers, which produced the following results: a. b. 333 red fruit, yellow flowers 64 red fruit, white flowers 58 orange fruit, yellow flowers 350 orange fruit, white flowers Conduct a chi-square analysis to demonstrate that these two genes DO NOT assort independently. Make sure to interpret the P value obtained from your chi-square test. Calculate and provide the map distance (in map units) between the two genes.arrow_forwardName: Date: Investigation: Is a dog more closely related to a coyote or a wolf? Gray Wolf Species Name: Canis lupus Color: Light gray to black Size: 80-120 pounds, 2.5 feet tall Appearance: broad snout, round ears, long tail Coyote Species Name: Canis latrans Color: Light gray to brown Size: 20-50 pounds, 1.5 feet tall Appearance: narrow snout, pointed ears, long tail Dog, Alaskan Malamute Species Name: Canis lupus familiaris Color: Gray and white or brown and white Size: 70-80 pounds, 2 feet tall Appearance: broad snout, round ears, long tail 1. Examine the images and descriptions above. Underline any similarities between the dog and the wolf. Place a star next to any coyote traits that are similar to the dog. 2. Based on appearance alone, which do you think is the most closely related to a dog? www.biologycorner.comarrow_forward
- yu yeuwyuyuierydtgcygucygzycghjcygyugfyudguygcywgduycgyudgs ygarrow_forwardAccording to a recent study, 1 out of 50,000 people will be diagnosed with cystic fibrosis. Cystic fibrosis can be caused by a mutant form of the CFTR gene (dominant gene symbol is F and mutant is f). A. Using the rate of incidence above, what is the frequency of carriers of the cystic fibrosis allele for CFTR in the US? (3 pts) B. In a clinical study, 400 people from the population mentioned in (A.) were genotyped for BRCA1 Listed below are the results. Are these results in Hardy- Weinberg equilibrium? Use Chi Square to show whether or not they are. (3 pts) # of women BRCA1 genotype BB 390 Bb bb 12pt v 10 0 V Paragraph B IUA BIUA > V T² v <arrow_forwardCase Study—Ella Ella has a family history of diabetes. She wants to follow a healthful eating pattern that can lower her risk for developing this condition. Her dietitian recommends a goal of 450 to 600 kcal per meal and advises Ella to follow the Acceptable Macronutrient Distribution Range (AMDR) for carbohydrates and the Dietary Guidelines for Americans 2015-2020, which recommend limiting added sugar. She also recommends that Ella choose whole grains rather than processed grains. Ella decides to pack a lunch to take to work every day. This morning she’s making a sandwich for her lunch. Categories of Sandwich Options (Top of the screen) Breads Spreads Cheeses Vegetables Proteins Specific food items to select White Bread 6-inches Honey Mustard Provolone LettuceTomatoBell Peppers Turkey Part A - Reading Nutrition Facts Panels for Total Kilocalories How many total kilocalories are in Ella’s sandwich? _______ kcal ? Part B - Reading Nutrition Facts Panels for…arrow_forward
- Case Study—Ella Ella has a family history of diabetes. She wants to follow a healthful eating pattern that can lower her risk for developing this condition. Her dietitian recommends a goal of 450 to 600 kcal per meal and advises Ella to follow the Acceptable Macronutrient Distribution Range (AMDR) for carbohydrates and the Dietary Guidelines for Americans 2015-2020, which recommend limiting added sugar. She also recommends that Ella choose whole grains rather than processed grains. Ella decides to pack a lunch to take to work every day. This morning she’s making a sandwich for her lunch. Categories of Sandwich Options (Top of the screen) Breads Spreads Cheeses Vegetables Proteins Specific food items to select White Bread 6-inches Honey Mustard Provolone LettuceTomatoBell Peppers Turkey Part A - Reading Nutrition Facts Panels for Total Kilocalories How many total kilocalories are in Ella’s sandwich exactl ______kcal ? Part B - Reading Nutrition Facts Panels for…arrow_forwardIn humans, red-green color blindness is recessive and X-linked, whereas albinism is recessive and autosomal. What types of children can be produced as the result of marriage between two homozygous parents, a normal-vision albino woman and a color-blind, normal male?arrow_forwardIn Drosophila, an X linked recessive mutation, scalloped (sd), causes irregular wing margins. Diagram the F1 and F2 results if a (a) scalloped female is crossed with a normal male; (b) a scalloped male is crossed with a normal female (assume the female is homozygous). Compare these results to what you would find if the trait was not sex linked.arrow_forward
- Case Study—Ella Ella has a family history of diabetes. She wants to follow a healthful eating pattern that can lower her risk for developing this condition. Her dietitian recommends a goal of 450 to 600 kcal per meal and advises Ella to follow the Acceptable Macronutrient Distribution Range (AMDR) for carbohydrates and the Dietary Guidelines for Americans 2015-2020, which recommend limiting added sugar. She also recommends that Ella choose whole grains rather than processed grains. Ella decides to pack a lunch to take to work every day. This morning she’s making a sandwich for her lunch. Categories of Sandwich Options (Top of the screen) Breads Spreads Cheeses Vegetables Proteins Specific food items to select White Bread 6-inches Honey Mustard Provolone LettuceTomatoBell Peppers Turkey Part A - Reading Nutrition Facts Panels for Total Kilocalories How many total kilocalories are in Ella’s sandwich exactl ______kcal ? Part B - Reading Nutrition Facts Panels for…arrow_forwardC MasteringHealth MasteringNu × session.healthandnutrition-mastering.pearson.com/myct/itemView?assignment ProblemID=17465255&attemptNo=1&offset=prevarrow_forwardBiopharmaceutics and Pharmacokinetics:Two-Compartment Model Instant Absorption Questions SHOW ALL WORK, including equation used, variables used and each step to your solution, report your regression lines and axes names (with units if appropriate) :Calculate a-q a) B1, b) B2, c) hybrid rate constant (1) d) hybrid rate constant (2) e) t1/2,dist f) t1/2,elim g) k10 h) k12 i) k21 j) initial concentration (C0) k) central compartment volume (V1) l) steady-state volume (Vss) m) clearance (CL) AUC (0→10 min) using trapezoidal rule n) AUC (20→30 min) using trapezoidal rule o) AUCtail (AUC360→∞) p) total AUC (using short cut method) q) volume from AUC (VAUC)arrow_forward

 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning





