
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 29.6, Problem BQ
Interpretation Introduction
Interpretation:
Among the given terms, the term that explains reason for addition of water to cyanoacrylate has to be identified.
Concept introduction:
Anionic Polymerizations: It can be initiated by addition of a nucleophile to an activated
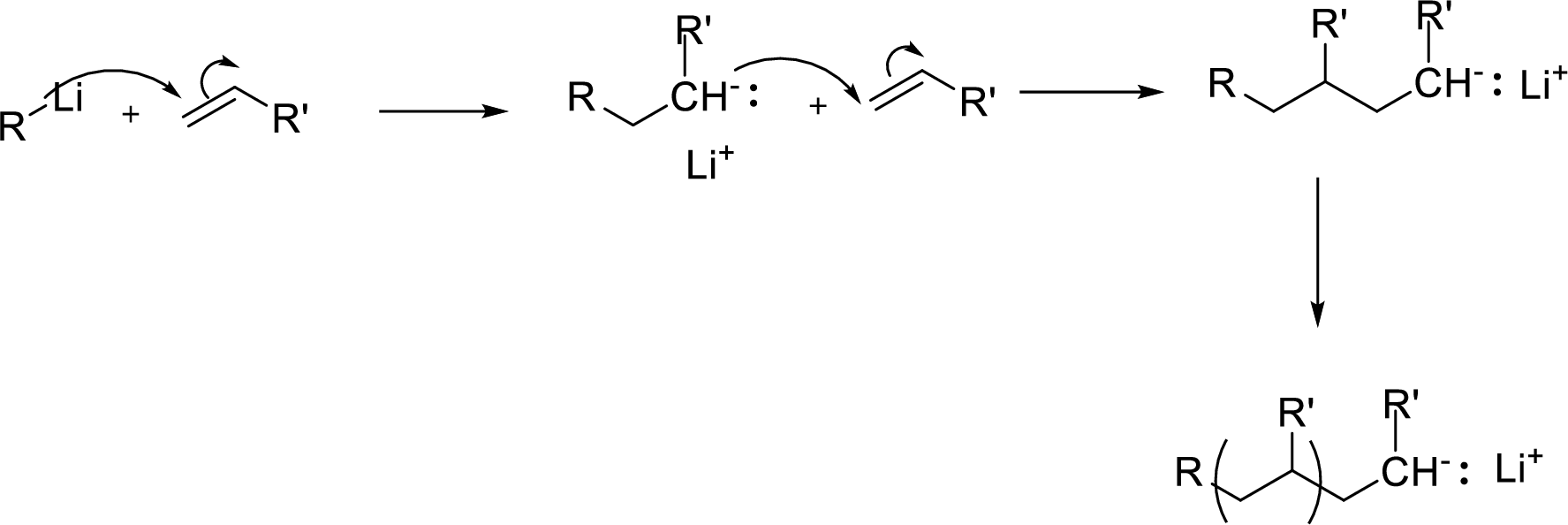
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The polymer shown below is synthesized by hydroxide ion-promoted hydrolysis of a copolymer of para-nitrophenyl methacrylate and acrylate. a. Propose a mechanism for the formation of the copolymer. b. Explain why hydrolysis of the copolymer to form the polymer occurs much more rapidly than hydrolysis of para-nitrophenyl acetate.
Why does methyl methacrylate not undergo cationic polymerization?
1) Which type of polymerization will the molecule shown most readily
undergo?
a.
Anionic
b. Cationic
C.
Radical
d. Ring-opening
e. Will not polymerize
Chapter 29 Solutions
Organic Chemistry
Ch. 29.2 - Prob. 29.1PCh. 29.5 - Prob. 29.2PCh. 29.6 - Prob. AQCh. 29.6 - Prob. BQCh. 29.6 - Prob. CQCh. 29.6 - Prob. DQCh. 29.6 - Prob. EQCh. 29.6 - Prob. FQCh. 29.6 - Prob. 29.3PCh. 29.6 - Prob. 29.4P
Ch. 29 - Prob. 29.5PCh. 29 - Prob. 29.6PCh. 29 - Prob. 29.7PCh. 29 - Prob. 29.8PCh. 29 - Prob. 29.9PCh. 29 - Prob. 29.10PCh. 29 - Prob. 29.11PCh. 29 - Prob. 29.12PCh. 29 - Prob. 29.13PCh. 29 - Prob. 29.14PCh. 29 - Prob. 29.15PCh. 29 - Prob. 29.16PCh. 29 - Polycarbonates (Section 29.5C) are also formed by...Ch. 29 - Prob. 29.18PCh. 29 - Prob. 29.19PCh. 29 - Prob. 29.20PCh. 29 - Prob. 29.21PCh. 29 - Draw a structural formula of the polymer resulting...Ch. 29 - Prob. 29.23PCh. 29 - Prob. 29.24PCh. 29 - Prob. 29.25PCh. 29 - Select the monomer in each pair that is more...Ch. 29 - Prob. 29.27PCh. 29 - Prob. 29.28PCh. 29 - Prob. 29.29PCh. 29 - Prob. 29.30PCh. 29 - Prob. 29.31PCh. 29 - Prob. 29.32PCh. 29 - Prob. 29.33PCh. 29 - Radical polymerization of styrene gives a linear...Ch. 29 - Prob. 29.35PCh. 29 - Prob. 29.36PCh. 29 - Prob. 29.37PCh. 29 - Prob. 29.38P
Knowledge Booster
Similar questions
- Radical polymerization of styrene gives a linear polymer. Radical polymerization of a mixture of styrene and 1,4-divinylbenzene gives a cross-linked network polymer of the type shown in Figure 29.1. Show by drawing structural formulas how incorporation of a few percent of 1,4-divinylbenzene in the polymerization mixture gives a cross-linked polymer.arrow_forward2.70 Why do you think an inhibitor molecule is needed to induce the polymerization of ethylene?arrow_forwardShow the structure of the polymer that results from heating the following diepoxide and diamine:arrow_forward
- Nylon 6 is a polyamide used in the manufacture of ropes. It can be prepared via hydrolysis of e-caprolactam to form e-aminocaproic acid followed by acid-catalyzed polymerization. & e-caprafactare 27.16a Hyd aminocaproic acid Identify the structure of Nylon 6. Nylonarrow_forward1) Select the appropriate reaction type for each of the following reactions. Nitration of Bromobenzene 1)+2) Grignard Reaction Polymerization Fischer Esterification Reduction of Benzoin Aldol Condensation Azo Dye Formation 1) + 2) Synthesis of Lidocaine A. Addition B. Bimolecular Nucleophilic Substitution C. Condensation D. Condensation Polymerization E. Electrophilic Aromatic Substitution F. Electrophilic Aromatic Substitution G. Nucleophilic Acyl Substitution Nucleophilic Addition on Carbonyl I. Protonation J. Unimolecular Elimination of c. Basearrow_forwardDraw a stepwise mechanism for the polymerization of isoprene to guttapercha using (CH3)3CO–OC(CH3)3 as the initiator.arrow_forward
- what is the mechanism of theses reactions? ( thermal polymerization)arrow_forwardThe anionic polymerization of ε-caprolactam. Even if the reaction rate between caprolactam is slow, the reaction with lactamanion and acylactam occurs fast. After drawing the structure of the product of the reactions below, explain the reason through the stability of the product.arrow_forwardShow the intermediate that would result if the growing chain added to the other end of the styrene double bond. Explain why the final polymer has phenyl groups substituted on every other carbon atom rather than randomly distributedarrow_forward
- the mechanism of the electrophilic additionarrow_forward1. Give the detailed arrow-pushing mechanism for the free radical polymerization of methyl methacrylate. Show the initiation step and the addition of at least 2 monomers. You do not need to show a termination step, but do show the [ Ja notation of the final polymer. NEATNESS COUNTS! **Attacn yuui TANarrow_forwarddraw a mechanism for the polymerization of caprolactam to nylon using curved arrows to show the flow of electrons and indicate whether it is a chain reaction or a step-growth reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning