
Concept explainers
(a)
Interpretation:
The given condition should be classified whether as a cause of respiratory or metabolic acidosis or alkalosis using the table 29.4.
Concept introduction:
The highly specific acid-base quality of various body fluid remains regulated within narrow limits because of high sensitivity of
Alkalosis is a term which refers to an increase in pH above the normal average of
Acidosis is a term which refers to an increase in
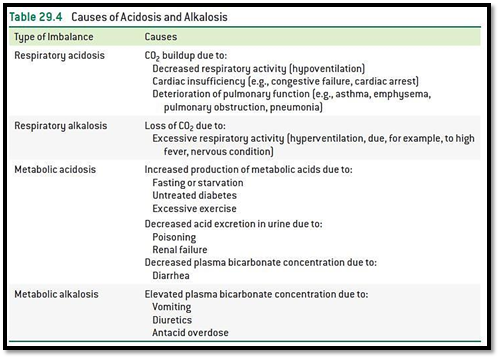
Figure 1
(b)
Interpretation:
The given condition should be classified whether as a cause of respiratory or metabolic acidosis or alkalosis using the table 29.4.
Concept introduction:
The highly specific acid-base quality of various body fluid remains regulated within narrow limits because of high sensitivity of metabolism to the
Alkalosis is a term which refers to an increase in pH above the normal average of
Acidosis is a term which refers to an increase in

Figure 1
(c)
Interpretation:
The given condition should be classified whether as a cause of respiratory or metabolic acidosis or alkalosis using the table 29.4.
Concept introduction:
The highly specific acid-base quality of various body fluid remains regulated within narrow limits because of high sensitivity of metabolism to the
Alkalosis is a term which refers to an increase in pH above the normal average of
Acidosis is a term which refers to an increase in

Figure 1
Want to see the full answer?
Check out a sample textbook solution
Chapter 29 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- Henry is in the intensive care unit because he suffered a severe myocardial infarction three days ago. The lab reports the followingvalues from an arterial blood sample: pH 7.30, HCO320 mEq/ liter, PCO2 32 mmHg. Diagnose Henry’s acid–base status and decide whether compensation is occurring.arrow_forward70. A 68-year-oldman with chronic respiratoryacidosis has an arterial pHof 7.34 and an arterial PCO2 of 55 mm Hg. Which of the following best explains the compensatory response to the acidosis? A) Decreased HCI secretion by the stomach B) Decreased tubular secretion of HCO," by the kidneys C) Increased CO2 excretion by the lungs O D) Increased HCO3" reabsorption by the kidneys E) IncreasedHCO,"transport by the choroid plexusarrow_forwardTable 1: Arterial blood gas concentration in patient Two hours after aspirin ingestion Ten hours after aspirin ingestion Normal values Partial Pressure CO2 26 mm Hg 19 mm Hg 35-45 mm Hg Partial Pressure O2 113 mm Hg 143 mm Hg 75-100 mm Hg Bicarbonate [HCO;] 18 mM 21 mM 22-26 mM pH 7.44 7.55 7.35-7.45 Blood salicylate concentration, mg/dL 57 117arrow_forward
- An individual's ratio of carbonic acid and bicarbonate ions is 17/0.6 Which of the following is a true statement? For a total of 3 answers - choose one of each of the following pairs: i) respiratory or metabolic ii) acidosis or alkalosis iii) uncompensated or partial compensationarrow_forwardCase Study: Bob is a 64-year-old male admitted to the emergency room for asthma. His laboratory results are as follows: pH 7.31, pCO2 higher than normal, and total HCO3– also higher than normal. Classify his acid-basebalance as acidosis or alkalosis, and as metabolic or respiratory. Is there evidence of compensation? Propose the mechanism by which asthma contributed to the lab results seen.arrow_forward1) Identify the lab value of Yusef's bicarbonate ion level in mEq/Liters. Only type in numbers, no letters (type out your number to zero places after the decimal point. i.e 8 or 18) pH = 7.68 PaCO2 = 21 mmHg 2) His condition is : 3 answers required: respiratory or metabolic acidosis or alkalosis uncompensated or partial compensation Group of answer choices respiratory metabolic acidosis alkalosis uncompensated partial compensationarrow_forward
- A patient exhibits the following blood characteristics. pH = 7.49 P co2 = 50 HCO3- = 29 mEq/L What is the likely condition? respiratory acidosis respiratory alkalosis metabolic acidosis metabolic alkalosisarrow_forwardA student got so excited about her acceptance into dental school that she began hyperventilating and then passed out. How might passing out, for a short time, be advantageous? What is her primary acid-base disorder?arrow_forwardThe following data was obtained from an arterial blood sample drawn from a hospital patient. pH= 7.55 (7.35-7.45) PaCO2=25mmHg (33-45mmHg) HCO3-= 22.5mEq/L (22-28mEq/L The patient’s arterial findings are consistent with a diagnosis of which of the following? metabolic acidosis respiratory alkalosis metabolic alkalosis mixed acidosis respiratory acidosisarrow_forward
- A patient suffers from hypokalaemia, and is prescribed an intravenous infusion of 0.15% potassium chloride (KCl) to correct this imbalance. She is to receive 50 mL 0.15% KCl over 4 hours. How many mmol/hour of potassium is she receiving? (0.15% KCl contains 20 mmol potassium per litre)arrow_forwardA patient presents to the emergency department unconscious, after ingesting a bottle of temazepam. What acid–base disturbance would you expect to see? Increased anion gap metabolic acidosis Respiratory alkalosis Normal gap metabolic acidosis Respiratory acidosisarrow_forwardThe immediate administration of nitrite is a highly effective treatment for cyanide poisoning. What is the basis for the action of this antidote? (Hint: Nitrite oxidizes ferrohemoglobin to ferrihemoglobin.)arrow_forward
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning

