
Concept explainers
ToDescribe:
The formation of the molecule of .
Answer to Problem 2Q
Solution:
The molecule can be formed by the reaction of limestone with hydrochloric acid.
Explanation of Solution
Calcium chloride occurs in nature in its hydrated form. The chemical formula of calcium chloride is . is an ionic compound and it is composed of calcium cation, that is, and two chlorine anions, that is, .
The calcium cation has a valency of two. Therefore, the calcium metal is bivalent. This bivalent calcium atoms forms an ionic bond with the two chlorine atoms.
The bond formation is shown below:
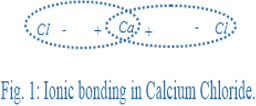
Given:
Chemical formula of Calcium Chloride, that is, .
Formula used:
Calculation:
Calcium chloride is formed by the chemical reaction between limestone, that is, and hydrochloric acid, that is, .
The chemical reaction is as follows:
Here, is Carbon dioxide and is water.
This is the equation showing the formation of .
Chapter 29 Solutions
Physics: Principles with Applications
Additional Science Textbook Solutions
The Cosmic Perspective (8th Edition)
Physics (5th Edition)
An Introduction to Thermal Physics
Conceptual Physics (12th Edition)
Conceptual Integrated Science
Introduction to Electrodynamics
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





