
(a)
Interpretation:
The reason for the isotope
Concept introduction:
The binding energy is defined as the energy required for breaking 1 mol of any nuclei into its individual nucleons.
Explanation of Solution
The graph is represented as follows:
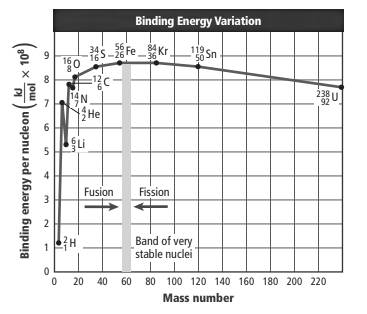
The above graph shows a change in binding energy per nucleon with an increase in mass numbers.
This can be seen from the graph that isotope
The binding energy is defined as the energy required breaking 1 mol of any nuclei into its individual nucleons. If the binding energy per nucleon is large, the force by which the nucleons are held together will be strong and the nucleus will be more stable. The binding energy per nucleon is lower for less stable atoms.
Here, the element with a mass number near 60 is most stable and it has maximum binding energy per nucleon.
(b)
Interpretation:
Whether more stable isotopes are located higher or lower on the curve needs to be determined.
Concept introduction:
The binding energy is defined as the energy required breaking 1 mol of any nuclei into its individual nucleons. If the binding energy per nucleon is large, the force by which the nucleons are held together will be strong and nucleus will be more stable. The binding energy per nucleon is lower for less stable atoms.
Explanation of Solution
The more stable isotopes have large binding energy per nucleons. The energy increases on moving from bottom to top in the graph thus, the isotopes located higher on the curve have maximum binding energy per nucleons thus, they are more stable isotopes.
(c)
Interpretation:
The stability of Li-6 and He-4 needs to be compared.
Concept introduction:
The binding energy is defined as the energy required breaking 1 mol of any nuclei into its individual nucleons. If the binding energy per nucleon is large, the force by which the nucleons are held together will be strong and nucleus will be more stable. The binding energy per nucleon is lower for less stable atoms.
Explanation of Solution
The binding energy curve is represented as follows:
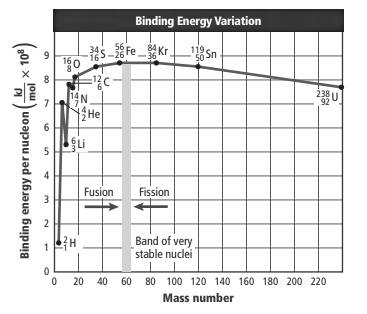
The points are represented for Li-6 and He-4. Here, mass number of Li is more than He but both are less than 60.
The binding energy per nucleon is less for Li-6 and it is approximately
Chapter 24 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: Structure and Properties (2nd Edition)
Organic Chemistry (8th Edition)
Chemistry: The Central Science (13th Edition)
Introductory Chemistry (6th Edition)
Chemistry: The Central Science (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





