
The crystal field stabilization energy (CFSE) can be defined as CFSE = 31 number of electrons the dxy, dxx, and dyzorbitals are stabilized (lower energy) with respect to the average energy of thed orbitals and that the deand dr, orbitals are destabilized. As described in Are You Wondering 24-3, the stabilization is -0.4
a. Plot the hydration energies as a function of the
b. Assuming that all the hexaaqua complexes are high spin, which ions have zero CFSE?
c. If lines are drawn between those ions with CFSE = 0, a line of negative slope is obtained. Can you explain this finding?
d. The ions that do not have CFSE = 0 have heats of hydration that are more negative than the lines drawn in part (c). What is the explanation for this?
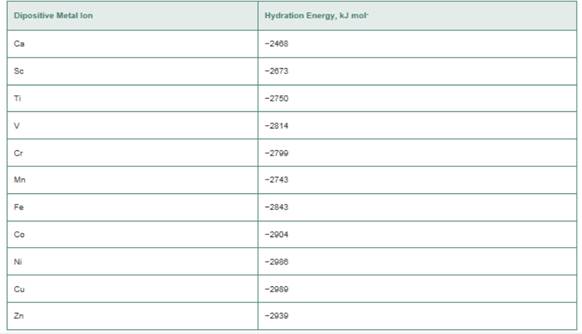
e. Estimate the value of L. for the Fe(ll) ion in an octahedral field of water molecules. What
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
- HO Hoor OH CF3 Br Br Ac₂O, pyr, DMAP COOH Bpin N3 cal Fe(OAc)2/ligand CN BnNH₂ Pd₂(dba)3 SPhos K3PO4 Cul, K₂CO3 cat. [Ir(cod)Cl]2 CI, MeOBIPHEP 3,4-(NO₂)BZOH CS₂CO3arrow_forwardeston 2 The complex (C u(NH,,"bb byht with a wamlength of 1,,1abserbs hight is the valus of A n K3 mele? 342 nm.Whatarrow_forwardThe coloriesS supe atanliquid was so 11. How does the addition of H3O* affect the following reaction equilibrium? 2CrO42- (aq, yellow) + 2H3O* (aq) -> Cr2O7²- (aq, orange) + 3H2O (1) a. The reaction shifts to the right and the concentration of CrO42- increases. b. The reaction shifts to the right causing the concentration of Cr2072 to decreases and the solution to turns yellow. c. The reaction shifts to the right resulting in a reduction in the concentration of H3O* and an increase in the concentration of Cr2O,2. d. The reaction shifts to the left and the concentration of CrO42 increases. e. The reaction shifts to the left and the concentration of CrO42 decreases.arrow_forward
- Q2/ Calculate the activation energy in( ev /atom) for vacancies formatiom of ( Al ) has ( 7.57 x 10") ( vacancy per m') at 500 e, atomic weight of (26.98) g/m, density of (2.62) g/em', (Vc) of (1) , avagadro number of (6.022 x 10), K of ( 8.62 x 10 ) ev/ atom-karrow_forwardThe factor determining the mechanism observed in the substitution reactions of square plane complexes is that these complexes are not saturated with coordination. Right?False?arrow_forwardksp for NiCO3 is 1.30 * 10 ^ -7arrow_forward
- An aqueous complex absorbs strongly at 528 nm. What color does the solution appear? 650 nm 580 nm Y 750 nm 560 nm 400 nm V 430 nm 490 nm O violet O yellow O blue O red O orange O greenarrow_forwardIn octahderal complexes, the Eigen- Wilkins mechanism explains the apparent disparity between the dependence of reaction rate on Y at low [Y] and the independence of reaction rate on Y at high [Y], by proposing the formation of an activated complex. O True O False Assuming the 18-electron rule, identify the metal M in [M(CO),(PPh)]. * PPH3 www ČO Cr O Mn O Fe Соarrow_forwardIn octahderal complexes, the Eigen- Wilkins mechanism explains the apparent disparity between the dependence of reaction rate on Y at low [Y] and the independence of reaction rate on Y at high [Y], by proposing the formation of an activated complex. O True O Falsearrow_forward
- Type of Chemial feactions: D 3NH4 NO, +YNQOH 4Na NO,+ 2NH3 + S H,0 2. C3 Hg +5 O, → 3 Co2 + 4 Hz20 3 pb(NO3 )2 +2KI →pb Iz +2KN Og D 2/2504+ NQ, Coz>2 NQHSO4 +H20 +Co, Ca ( Cs Hz Oz)2+ Naz (C, O4)>2Na(GH3 O2)+Ca (GHOarrow_forwarddraw the orbital overlap between transition metal d-orbitals and an oxo p-orbitals to form a triple bond and using an orbital energy diagram, determine the bond order of a Mn2+-O complex and a Ta3+-O complex; and state if we have lost our ‘yl-oxo’ bond or not inorganic chemarrow_forwardX and Y form a complex whose fluorescence can be measured. Use the following data to determine the concentration of X in the sample. The concentration of X in the standard is 4.15 x 10-³ M. Vx,sample (ml) Vy(ml) Vs.standard (ml) VH20(ml) Rel. F Solution 1 3.0 3.0 0.0 3.0 13.8 Solution 2 3.0 3.0 2.0 1.0 34.3 a. none of the other answers are correct 4.90 x 10-5 M 10-5 M С. 1.86 x d. 7.47 x 10-6 M 10-4 M е. 6.76 xarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

