
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
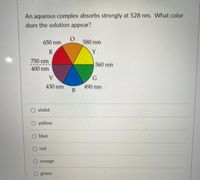
Transcribed Image Text:**Question:**
An aqueous complex absorbs strongly at 528 nm. What color does the solution appear?
**Visual Representation:**
The image includes a color wheel illustrating the relationship between wavelength (in nanometers) and color:
- **Red (R):** 650 nm
- **Orange (O):** 580 nm
- **Yellow (Y):** 560 nm
- **Green (G):** 490 nm
- **Blue (B):** 430 nm
- **Violet (V):** 400 nm to 750 nm
**Multiple Choice Options:**
- ○ Violet
- ○ Yellow
- ○ Blue
- ○ Red
- ○ Orange
- ○ Green
**Explanation:**
The color wheel in the image demonstrates which colors correspond to specific wavelengths. When a solution absorbs light at 528 nm (near green), it will most likely appear red due to the complementary color relationship typically observed in colorimetry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the concentration of free Hg2+ in 2.6061e-4 M Hg(NO3)2 and 1.1005 M NH3? Hg2+ + 4 NH3 → [Hg(NH3)4]2+ Kf = 1.800e+19arrow_forwardA ligand (L) binds to a protein with a Kd value of 0.1x10-6 M. What percentage (%) of the proteins are bound if the free ligand concentration was measured at 100 nM?arrow_forwardWhich of the following is a pair of diastereomers? Br Br Br Br ČI CI %3D III IV O l and III O Il and III O II and IV O Il and IVarrow_forward
- !5arrow_forwardAt pH 1, superoxide ions are is largely present in water as? О НО2 O H202" O HO22- O 02 O H2022+ QUESTION 2 Au(1) complexes are aurophilic and isoelectronic with O Hg(II) O Pt(II) O Pd(1) O Ir(III) O Rh(I)arrow_forwardThe red color of soil is often due to the presence of iron. Metal ions are extracted from soil by stirring the soil in acid and then filtering the solution. One method for the analysis of Fe2+ is to form the highly colored Fe2+–thioglycolic acid complex. The complex absorbs strongly at 535 nm. Calibration standards of 1.00, 2.00, 3.00, 4.00, and 5.00 ppm are prepared by transferring appropriate amounts of a 10.0 ppm working solution of Fe2+ into separate 50-mL volumetric flasks, each of which contains 5 mL of thioglycolic acid, 2 mL of 20% w/v ammonium citrate, and 5 mL of 0.22 M NH3. After diluting to volume and mixing, the absorbances of the standards are measured. a)Use the data table below to prepare a calibration curve (absorbance versus concentration in ppm). Fit the data to straight line and find the equation for the straight line and the R2 value. (Hint: Think about what to do with the absorbance of the blank.)arrow_forward
- Which one of the following substances is the product of this co -- (s) 1 + (s) IVarrow_forwardYou measured the absorbance values at selected wavelengths for one of the complexes above. Record those values below, as well as the identity of your chosen complex. Determine your estimate for the wavelength of maximum absorbance (λmax), and show your calculations for the energy of d-orbital splitting, Δ. 0.10 M Ni(NO)2 : 10 drops approximately 0.50 mL Chosen complex: (Ethylenediamine) C2H4(NH2)2 + Ni(NO)2 (10 drops, hot temperature, light purple color) Wavelength (λ) nanometers Absorbance 400 nm 0.438 450 nm 0.047 500 nm 0.094 550 nm 0.184 600 nm -0.043 650 nm -0.066 700 nm -0.0125 750 nm -0.121 800 nm -0.218arrow_forwardWhat is the NAME for the following ion? NO2 H₂ H₂ -N N Co. N₂ NO₂ mer-dinitrobis(ethylenediamine)cobaltate(III) trans-bis(ethylenediamine)dinitrocobalt(III) cis-dinitrobis(ethylenediamine)cobaltate(III) fac-di(ethylenediamine)dinitrocobalt(III) cis-di(ethylenediamine)dinitrocobalt(III)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY