
Concept explainers
(a)
Interpretation:
The number of acetyl CoA is formed from complete beta-oxidation of the myristic acid, needs to be identified.
Concept Introduction:
Beta − oxidation of fatty acids involves four series of reactions. In this reaction, the long-chain fatty acid is degraded into many two-carbon unit's acetyl CoA.
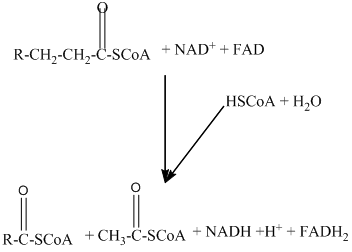
The number of acetyl CoA molecules formed, and number of time beta-oxidation occur can be determine by using the number of carbon atoms present in the fatty acid.
(b)
Interpretation:
The number of cycles of beta-oxidation is required for complete oxidation needs to be identified.
Concept Introduction:
Beta − oxidation of fatty acids involves four series of reactions. In this reaction, the long-chain fatty acid is degraded into many two-carbon unit's acetyl CoA.
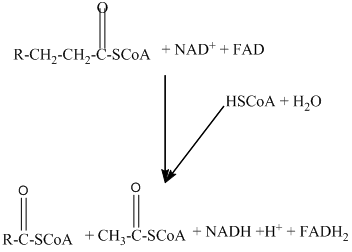
The number of acetyl CoA molecules formed, and the number of time beta-oxidation occur can be determined by using the number of carbon atoms present in the fatty acid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
General, Organic, and Biological Chemistry - 4th edition
- (a) Give the omega designation for the following fatty acid: Но (b) Give the omega designation for the following fatty acid: HOarrow_forward4 Draw the structure of an example of each of the following types of lipids:(a) a saturated fat (b) a polyunsaturated oil (c) a wax(d) a soaparrow_forwardWhy might some food companies find it economically advantageous to advertise their products (such as corn oil and margarine) as being composed of polyunsaturated fatty acids?arrow_forward
- Give the omega designation for the following fatty acid:arrow_forwardWater production from fatty acid oxidation is a survival mechanism in animals where water is scarce. Cells oxidize palmitoyl-CoA to produce water. If an animal has 5.000 kg of palmitoyl-CoA available for fatty acid oxidation, how many kilograms of water could the animal produce from that palmitoyl-CoA? The chemical formula for palmitoyl-CoA is C37H66N7O17P3S.arrow_forwardDefine the Hydrogenation of unsaturated fatty acids ?arrow_forward
- GLYCOLYSIS: 1A) Starting with glucose (in the open-chain Fisher projection), draw out the molecular structures for each step of glycolysis. For each step, include the name of the enzyme that catalyzes the reaction. 1B) What is the net reaction of glycolysis? CITRIC ACID CYCLE: 2A) Starting with acetyl-coenzymeA and oxaloacetate, draw out the molecular structures for each step of the citric acid cycle. For each step, include the name of the enzyme that catalyzes the reaction. 2B) What is the net reaction of the citric acid cycle? What happens to each product? OXIDATIVE PHOSPHORYLATION: 3A) Thoroughly explain the biological significance of NADH/H* and FADH2 and their roles in oxidative phosphorylation. 3B) Describe and diagram the general pathway of the electrons from NADH/H+ and FADH₂ to O₂ during the electron transport chain. 3C) What is "chemiosmotic coupling", and what is its relationship to ATP synthesis? 3D) During oxidative phosphorylation, what is oxidized and what is…arrow_forwardOxaloacetic acid (or 2‑ketosuccinic acid) is a very important intermediate in metabolism. The compound is involved in the citric acid cycle for energy production within the cell. However, the compound is unstable and slowly decomposes spontaneously. Draw the decomposition products.arrow_forwardYou are given 150 mL of an oil made up of a mixture of 3 fatty acids: palmitoleic acid (C 16:1 (n-7)) erucic acid (C 22:1 (n-9)) stearidonic acid (C 18:4 (n-3)) You know that the 150 mL sample of this oil was prepared by adding 60 mL of palmitoleic acid to 90 mL of a mixture of erucic acid and stearidonic acid. Using a hydrometer you carefully measure the density of the 150 mL sample of oil and your analysis indicates that it has a density of 0.900 g/cm3. The densities of the individual fatty acids are: palmitoleic acid = 0.894 g/cm3 erucic acid = 0.860 g/cm3 stearidonic acid = 0.9334 g/cm3 Can you determine the volumes (in mL) of erucic acid and stearidonic acid in the total volume (150 mL) of the oil mixture?arrow_forward
- Give the over-all chemical equation of the complete oxidation of stearic acid (an 18-C fatty acid).arrow_forwardThe formation of a thiol ester makes o a) ATP ob) NADH oc) Acetyl COA od) Coenzyme A QUESTION 5 is a polymer of a) Phospholipid; fatty acids b) Cholesterol; isoprene units c) A wax; fatty acids od) Sphingolipid; sphingosine a high energy compound. QUESTION 6 In the glycolysis pathway, 1,3 bisphosphoglycerate is converted to 3-phosphoglycerate via a phosphate transfer reaction in which ATP is formed from ADP. The hydrolysis of a bond provides the energy to produce this ATP. o a) phosphate ester ob) acylphosphate o c) phosphoanhydride d) thioesterarrow_forwardDraw the product of the complete hydrogenation (reduction) of the following fatty acid: -HO-arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



