
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 23, Problem 2PP
Interpretation Introduction
Interpretation:
The isoprene units in the given compounds are to be shown and each of them as per their isoprene units are to be classified.
Concept introduction:
Essential oils are mixtures of odoriferous compounds that are extracted from plants by heating or steam distillation. These essential oils have important chemical composition of hydrocarbons called terpenes and oxygen-containing compounds called terpenoids.
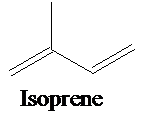
A single isoprene unit consists of 5-carbon atoms with double bonds, and its chemical formula is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) Show the isoprene units in each of the following terpenes.(b) Classify each as a monoterpene, sesquiterpene, terpene,etc.
Propose structure and give correct IUPAC names for the following:
(a) A cyclic alkane with three methyl groups
(b) A diethyldimethylhexane
Biphenyl has the following structure.(a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon?(b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?(c) The heat of hydrogenation for biphenyl is about 418 kJ>mol (100 kcal>mol). Calculate theresonance energy of biphenyl.(d) Compare the resonance energy of biphenyl with that of naphthalene and with that of two benzene rings. Explain thedifference in the resonance energies of naphthalene and biphenyl.
Chapter 23 Solutions
Organic Chemistry
Ch. 23 - Prob. 1PPCh. 23 - Prob. 2PPCh. 23 - Prob. 3PPCh. 23 - Prob. 4PPCh. 23 - Prob. 5PPCh. 23 - Prob. 6PPCh. 23 - Prob. 7PPCh. 23 - Prob. 8PPCh. 23 - Prob. 9PPCh. 23 - Prob. 10PP
Ch. 23 - Prob. 11PPCh. 23 - Prob. 12PPCh. 23 - Prob. 13PPCh. 23 - Prob. 14PCh. 23 - 23.15 How would you transform tetradecanal into...Ch. 23 - Prob. 16PCh. 23 - Prob. 17PCh. 23 - When limonene (Section 23.3) is heated strongly,...Ch. 23 - Gadoleic acid (C20H38O2), a fatty acid that can be...Ch. 23 - 23.20 -Phellandrene and -phellandrene are isomeric...Ch. 23 - Prob. 21PCh. 23 - Prob. 22PCh. 23 - Prob. 23PCh. 23 - The initial steps of a laboratory synthesis of...Ch. 23 - Prob. 25PCh. 23 - Prob. 26PCh. 23 - Prob. 27PCh. 23 - 2. The biosynthesis of fatty acids is accomplished...Ch. 23 - Prob. 3LGPCh. 23 - Prob. 4LGP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose structures and give IUPAC names for the following: (a) A diethyldimethylhexane (b) A (3-methylbutyl)-substituted alkanearrow_forward(a) Compound D undergoes a reaction with hydrogen bromide, HBr to produce 2-bromobutane. D exists as cis-trans isomers and decolourises bromine solution in methylene chloride, CH2CI2. (i) Draw and name the structure of compound D. (ii) Draw two (2) constitutional isomers of compound D.arrow_forward26(a) Name the following compound.arrow_forward
- (a) One test for the presence of an alkene is to add a smallamount of bromine, which is a red-brown liquid, and lookfor the disappearance of the red-brown color. This test doesnot work for detecting the presence of an aromatic hydrocarbon.Explain. (b) Write a series of reactions leading topara-bromoethylbenzene, beginning with benzene andusing other reagents as needed. What isomeric side productsmight also be formed?arrow_forward(a) Give chemical tests to distinguish between compounds in the following pairs of substances :(i) Ethanol and Propanal (ii) Benzoic acid and Ethyl benzoate(b) An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acids. Derive the structure of the compound.arrow_forwardAnswer ALL parts of this question. (a) Provide the structure and name of the aldehyde with the formula, C2H4O. (b) Draw the two steps of the Strecker synthesis used for the conversion of the aldehyde in part (a) into racemic alanine. (c) Draw the (R) and (S) enantiomers of alanine. Which is the naturally occurring enantiomer? (d) Draw the structure of the dipeptide, Ala-Gly.arrow_forward
- (a) Differentiate between copolymerization and homopolymerization. Give one example of each.(b) What is the role of Benzoyl peroxide in preparation of Polythene?arrow_forwardBiphenyl has the following structure.(a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon?(b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?arrow_forwardAspirin, or 2-acetoxybenzoic acid, (C9H8O4) is often synthesised from salicylic acid.(i) Sketch and discuss any changes in the number of possible structural conformations ofaspirin relative to those of salicylic acid. (ii) Re-draw the structure predicted to be the lowest energy conformation of aspirin,indicating any expected stabilising and destabilising interactions. Justify your choice.arrow_forward
- Explain why (i) the dipole moment in chlorobenzene is lower than that of cyclohexyl chloride. (ii) haloalkanes are only slightly soluble in water but dissolve easily in organic solvents.arrow_forwarda) Give the chemical structure for each of the following compound: (i) 4-hydroxybenzoic acid (ii) 2-hexene (iii) chlorocyclohexane (iv) 2,5-dichloroheptane b) Prostaglandin are a group of C20 lipids and can be found in small amounts in all body tissues and fluids. (1) Put an asterisk on all the chiral carbon(s) present in the chemical structure of prostaglandin E. (II) Do you expect prostaglandin to be acidic or basic? (III) Circle the functional group that is responsible for the acidity / basicity. ali se odno HO OH HO Prostaglandin Earrow_forwardShow the chemical reaction on how to convert cyclopentene into these compounds. (a) 1,2-dimethylcyclopropane (b) Cyclopentanol (c) Iodocyclopentane (d) Cyclopentane.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY