
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 23, Problem 28P
Pentafluorophenol is readily prepared by heating hexafluorobenzene with
potassium hydroxide in tert-butyl alcohol:
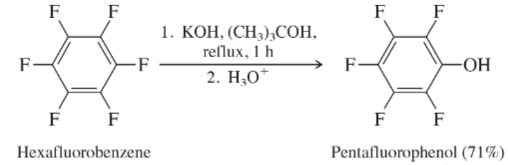
What is the most reasonable mechanism for this reaction? Comment on the comparative ease with which this conversion occurs.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Compounds with more than one hydroxyl group can react with thionyl chloride differently from simple alcohols. In the reaction with thionyl chloride, the butane-1,2-diol gave a single organic product with 85% yield, according to the following balanced equation:
(Suggest a reasonable structure for this product and make a mechanism to justify your reasoning using curved arrows to describe the motion of electrons)
When bromine is added to two beakers, one containing phenyl isopropyl ether and the other containing cyclohexene, the bromine color in both beakers disappears. What observation could you make while performing this test that would allow you to distinguish the alkene from the aryl ether?
An ester W with a molecular formula of C6H12O2 is optically active. When W is undergone acidic hydrolysis, compound X, C2H4O2 and an alcohol Y are formed. Alcohol Y is optically active and gives a yellow precipitate with alkaline iodine. Oxidation of Y produces Z which is optically inactive
Identify compounds W, X, Y and Z.
Write balanced equations for the formation of alcohol Y.
Other than using alkaline iodine, suggest how you would differentiate between X and Y.
State how Z reacts (if any) with,
Alkaline iodine
2,4-dinitrophenylhydrazine
Tollen’s reagent
Chapter 23 Solutions
Organic Chemistry - Standalone book
Ch. 23.1 - Prob. 1PCh. 23.3 - Problem 23.2 One of the hydroxybenzoic acids is...Ch. 23.4 - Prob. 3PCh. 23.5 - Prob. 4PCh. 23.6 - Problem 23.5 The compound shown was required for...Ch. 23.8 - Prob. 6PCh. 23.8 - Prob. 7PCh. 23.9 - Prob. 8PCh. 23.9 - Prob. 9PCh. 23.10 - Prob. 10P
Ch. 23.12 - Prob. 11PCh. 23 - The IUPAC rules permit the use of common names for...Ch. 23 - Prob. 13PCh. 23 - Prob. 14PCh. 23 - Prob. 15PCh. 23 - Prob. 16PCh. 23 - Prob. 17PCh. 23 - Prob. 18PCh. 23 - Prob. 19PCh. 23 - Prob. 20PCh. 23 - Prob. 21PCh. 23 - Prob. 22PCh. 23 - Prob. 23PCh. 23 - Prob. 24PCh. 23 - Prob. 25PCh. 23 - Prob. 26PCh. 23 - Choose the reaction in each of the following pairs...Ch. 23 - Pentafluorophenol is readily prepared by heating...Ch. 23 - Prob. 29PCh. 23 - Treatment of p-hydroxybenzoic acid with aqueous...Ch. 23 - Prob. 31PCh. 23 - Prob. 32PCh. 23 - Treatment of 2,4,6-tri-tert-butylphenol with...Ch. 23 - Prob. 34PCh. 23 - Prob. 35PCh. 23 - Prob. 36DSPCh. 23 - Prob. 37DSPCh. 23 - Prob. 38DSPCh. 23 - Prob. 39DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Photochemical chlorination of 2,2,4-trimethylpentane gives four isomeric monochlorides. (a) Write structural formulas for these four isomers. (b) The two primary chlorides make up 65% of the monochloride fraction. Assuming that all the primary hydrogens in 2,2,4-trimethylpentane are equally reactive, estimate the percentage of each of the two primary chlorides in the product mixture.arrow_forwardDescribe how would you distinguish the following pairs, (a) Benzene and cyclohexane (b) Phenol and toluene (c) Phenol and benzoic acid (d) methanol and isopropyl alcoholarrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent ofH2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragmentis propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are thestructures of A, B, and C? Write all reactions and show your reasoning.arrow_forward
- Must stereochemistry be considered when synthesizing ethylene glycol from ethene? Explain.arrow_forwardAlkenes can be converted to alcohols by reaction with mercuric acetate to form a β-hydroxyalkylmercury(II) acetate compound, a reaction called oxymercuration. Subsequent reduction with NaBH4 reduces the C–Hg bond to a C–H bond, forming the alkyl alcohol, a reaction called demercuration. Draw the structures of the Hg-containing compound(s) and the final alcohol product(s) formed in the following reaction sequence, omitting byproducts. If applicable, draw hydrogen at a chirality center and indicate stereochemistry via wedge-and-dash bonds.arrow_forwardSuggest a method of preparing ethyl benzene, starting with benzene and ethylene as the only organic reagents.arrow_forward
- (a) Write a chemical test to distinguish between: (i) Chlorobenzene and Benzyl chloride. (ii) Chloroform and Carbon tetrachloride. (b) Why is methyl chloride hydrolysed more easily than chlorobenzene?arrow_forwardYou are asked to prepare (synthesize) chloroethane using an alkane and any inorganic substance of your choice. Write a chemical equation for the synthesis of chloroethane. 3.arrow_forwardGiven the chemical equation in the synthesis of benzoic acid from toluene and potassium permanganate: C7H8(I) + 2KMnO4(aq) → KC7H5O2 + 2MnO2 (s) + KOH (aq) + H2O (I) KC2H5O2 (aq) + HCl (aq) → C7H8O2 (s) + KCl (aq) MW: C7H8 = 92.14 KMnO4 = 158.03 MnO2 = 86.94 KC7H5O2 = 160.21 C7H8O2 = 122 Density: C7H8 = 0.87 g/mL KMnO4 = 2.7 g/mL KC7H5O2 = 1.5 g/mL C7H8O2 = 1.27 g/mL If 1 mL of toluene and 4 mL of 50% w/v potassium permanganate solution are used to synthesize the benzoic acid in a reflux set-up, calculate the theoretical yield of benzoic acid. Show your complete solution and underline your final answer.arrow_forward
- A compound with formula C7H12O is treated with sodium borohydride in methanol to yield 2,2-dimethylcylopentanol. Write a reaction scheme showing the structures of the reactant, the reagents, and the product. Will the product be optically active? Explain.arrow_forwardIsoamyl acetate is the common name of the substance most responsible for the characteristic odor of bananas. Write a structural formula for isoamyl acetate, given the information that it is an ester in which the carbonyl group bears a methyl substituent and there is a 3-methylbutyl group attached to one of the oxygens.arrow_forwardWrite the structural formulas for two (2) chloro-isomers that can formed from 2-methypropane (isobutene). How many sets of equivalent hydrogen are there in the compound 2-methypropane?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY