
Concept explainers
(a)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(a)
Answer to Problem 22.59QP
The d-electrons are distributed in the complex ion

The complex has square planar geometry.
Explanation of Solution
In the complex ion

The distribution of d-electrons as shown above correlates to that of square planar geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(b)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(b)
Answer to Problem 22.59QP
The d-electrons are distributed in the complex ion

The complex has square planar geometry.
Explanation of Solution
In the complex ion
Atomic number of Cobalt is

The distribution of d-electrons as shown above correlates to that of square planar geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(c)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(c)
Answer to Problem 22.59QP
The d-electrons are distributed in the complex ion
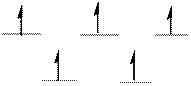
The complex has tetrahedral geometry.
Explanation of Solution
In the complex ion
Atomic number of Iron is

The distribution of d-electrons as shown above correlates to that of tetrahedral geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(d)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(d)
Answer to Problem 22.59QP
The d-electrons are distributed in the complex ion
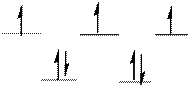
The complex has tetrahedral geometry.
Explanation of Solution
In the complex ion
Atomic number of Cobalt is

The distribution of d-electrons as shown above correlates to that of tetrahedral geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
Want to see more full solutions like this?
Chapter 22 Solutions
General Chemistry - Standalone book (MindTap Course List)
- How will increasing the temperature by ten degrees Celsius impact the rate of reaction? Select the correct answer below: the rate will double the rate will quadruple the rate will be cut in half depends on the reactionarrow_forwardShow work. Don't give Ai generated solution. Don't give Handwritten answerarrow_forwardShow work...arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





