
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 21.4, Problem AQ
Interpretation Introduction
Interpretation:
The other functional group is identified in Capsaicin other than phenol.
Concept introduction:
Functional group: They are certain substitutes in the organic molecules which are determine the characteristic reactions taking place in it.
Amide:
A carbon atom is double-bonded to an oxygen atom
If the carbonyl carbon is attached with nitrogen is called as amide.
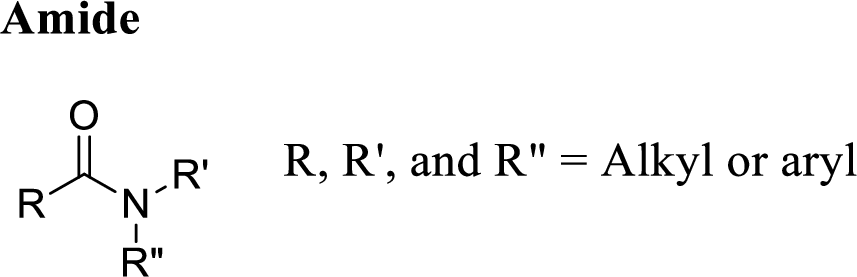
Alkenes are a class of hydrocarbons. The carbon-carbon double bond is called as alkenes and it is also called as olefins.
Ether:
The aliphatic or
Example is given below
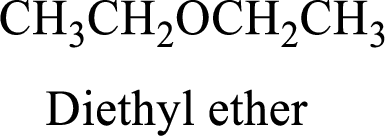
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Amide hydrolysis in basic conditions forms
A. a carboxylic acid and an amine
B. a carboxylate salt and an amine
3. an ester and an amine
4. a carboxylic acid and an amine salt
1. Which of the following statements is true?
I. Aldehydes and ketones both contain a hydroxyl group.
II. The names for aldehydes and ketones are derived from the name of the longest carbon chain that contains the carbonyl group.
III. The aldehyde and ketone with a molecular formula of C3H6O are constitutional isomers.
IV. 2-Propanone is immiscible in water.
A. I & II
B. II & II
C. I & III
D. I & IV
2. Whicb of the following is the correct IUPAC name of the structure below?
in
medicine as the first line of defence against breast cancer. Identify ALL the
different functional groups in the molecule.
оно
NH
ÖH O
A. Aromatic ring, carboxylic acid, ester, amide, alkene, alcohol, cyclic ether.
B. Substituted benzene ring, amide, alcohol, ester, alkene, ketone, cyclic ether.
C. Substituted benzene ring, carboxylic acid, ester, amide, alkene, alcohol,
aldehyde.
D. Aromatic ring, ketone, cyclic ester, amide, alkene, alcohol, aldehyde.
E. Aromatic ring, aldehyde, ester, amide, alkene, alcohol, acyclic ether.
Chapter 21 Solutions
Organic Chemistry
Ch. 21.2 - Construct a Frost circle for a planar...Ch. 21.2 - Which compound gives a signal in the 1H-NMR...Ch. 21.2 - Prob. 21.3PCh. 21.2 - Prob. 21.4PCh. 21.3 - Prob. 21.5PCh. 21.4 - Arrange these compounds in order of increasing...Ch. 21.4 - Prob. AQCh. 21.4 - Prob. BQCh. 21.4 - Prob. CQCh. 21.5 - Prob. 21.7P
Ch. 21 - Name the following compounds and ions.Ch. 21 - Prob. 21.9PCh. 21 - Draw a structural formula for each compound. (a)...Ch. 21 - Molecules of 6,6-dinitrobiphenyl-2,2-dicarboxylic...Ch. 21 - Following each name is the number of Kekul...Ch. 21 - Prob. 21.13PCh. 21 - Prob. 21.14PCh. 21 - Prob. 21.15PCh. 21 - Which of the molecules and ions given in Problem...Ch. 21 - Prob. 21.17PCh. 21 - Naphthalene and azulene are constitutional isomers...Ch. 21 - Prob. 21.19PCh. 21 - Prob. 21.20PCh. 21 - Following are IR and 1H-NMR spectra of compound D....Ch. 21 - Compound E (C8H10O2) is a neutral solid. Its mass...Ch. 21 - Following are 1H-NMR and 13C-NMR spectral data for...Ch. 21 - Following are 1H-NMR and 13C-NMR spectral data for...Ch. 21 - Compound H (C8H6O3) gives a precipitate when...Ch. 21 - Compound I (C11H14O2) is insoluble in water,...Ch. 21 - Propose a structural formula for compound J...Ch. 21 - Propose a structural formula for the analgesic...Ch. 21 - Prob. 21.29PCh. 21 - Prob. 21.30PCh. 21 - Given here are 1H-NMR and 13C-NMR spectral data...Ch. 21 - Prob. 21.32PCh. 21 - Prob. 21.33PCh. 21 - Prob. 21.34PCh. 21 - Arrange the molecules and ions in each set in...Ch. 21 - Prob. 21.36PCh. 21 - Prob. 21.37PCh. 21 - From each pair, select the stronger base.Ch. 21 - Prob. 21.39PCh. 21 - Prob. 21.40PCh. 21 - Prob. 21.41PCh. 21 - Prob. 21.42PCh. 21 - Following is an equation for iodination of...Ch. 21 - Prob. 21.44PCh. 21 - Prob. 21.45PCh. 21 - Prob. 21.46PCh. 21 - When warmed in dilute sulfuric acid,...Ch. 21 - In the chemical synthesis of DNA and RNA, hydroxyl...Ch. 21 - Prob. 21.49PCh. 21 - Write the products of the following sequences of...Ch. 21 - Prob. 21.51PCh. 21 - Show how to convert 1-phenylpropane into the...Ch. 21 - Prob. 21.53PCh. 21 - Cromolyn sodium, developed in the 1960s, has been...Ch. 21 - Prob. 21.55PCh. 21 - Prob. 21.56PCh. 21 - Prob. 21.57PCh. 21 - Prob. 21.58PCh. 21 - Prob. 21.59PCh. 21 - Prob. 21.60PCh. 21 - Prob. 21.61PCh. 21 - Prob. 21.62PCh. 21 - Prob. 21.63PCh. 21 - Following is a synthesis for toremifene, a...
Knowledge Booster
Similar questions
- 2. Oxybenzone and 2-ethylhexyl-p-methoxycinnamate are UV absorbing compounds used in sunscreens. Identify and encircle the functional groups in these compounds. a. Oxybenzone Ö HÓ b. 2-ethylhexyl-p-methoxycinnamatearrow_forwardWhat are the major products of the reaction of ethyl benzoate with hydrochloric acid and water? a. acetic acid and toluene b. phenylic acid and ethanol C. ethanoic acid and benzene d. benzoic acid and ethanol e. phenylic acid and methanol O a O barrow_forwardamine, (2) an amide, or (3) both an amine and an amide. 17-106 Classify each of the following compounds as (1) an amine, (2) an amide, or (3) both an amine and an NH2 b. `NH a. H2N H d. с.arrow_forward
- 4. Tylenol also is an analgesic often taken by people allergic to aspirin. The active ingredient is acetaminophen. HO- -NH H3C Would acetaminophen give a positive phenol test? (Y/N). Does this compound contain the ester functional group? If not, what functional group(s) is/are present?arrow_forwardSelect all functional groups present in the following structure of a drug commonly found in a dentist's office N a) Alkyl halide b) Alcohol c) Carboxylic acid d) Phenol e) Amine f) Ether Og) Amide h) Ester i) Ketone 0 j) Aromatic ring (aka phenyl) (aka arene)arrow_forward14. PART 3: Draw the structure for compound A.arrow_forward
- 2. What is produced when an amine reacts with water? A. A primary alcohol and ammonia B. An amide and a hydrogen (H+) ion C. An ammonium ion and a hydroxide (OH-) ion D. An amide and a hydroxide (OH-) ionarrow_forward16. An atom or group of atoms that can give organic compounds distinct chemical and physical properties. 21. When a compound with the general formula R-COOH loses a proton, the product that remains is described with this term. Its general formula is R-COO- 24. A class of organic compounds in which three or more carbons form a ring structure. All of the carbon-to-carbon bonds are single bonds in this family of compounds.arrow_forward4. The organic starting materials for the preparation of an ester could be C. a ketone and alcohol A an acid and alcohol B. water and oxygen D. alkane and aldehydearrow_forward
- 2. a. What does this product smell like? b. What natural product does it come from?arrow_forward1. Amines are classified according to the number of alkyl or aryl groups directly attached to a certain atom in the molecule. What atom is this? 2. Draw examples of a primary, secondary, and tertiary amine. Secondary Primary H N-CH3 Tertiary CHS N-CH3 CH CHa N-CHS 3. What are the names of CH3CH2-NH, in the IUPAC and common naming systems? 4. Complete the following equation and name the organic reactants and products. CH,NH, (aq) + H,O = 5. What is the general name of the product of the neutralization reaction between an alkylamine and an acid? AIKVI Ammonium Salt laarrow_forwardA lot of controversy surrounds the use of Aspartame as an artificial sweetener. The main argument is concerned with the production of methanol in the body. From the structure of aspartame given below: G0₂CH3 HẠN CH—ỆNH—CHCH, CH, COCH Source for the formation of methanol is, A. ester group in the compound B. amide group in the compound C. carboxylic acid group in the compound D. amino group in the compound E. benzene ring in the compoundarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning