
(a)
Interpretation:
The synthesis of carbinoxamine has to be shown.
Concept introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (RMgX) is called as Grignard reagent.
Grignard reagent is reaction with carbonyl compound such as
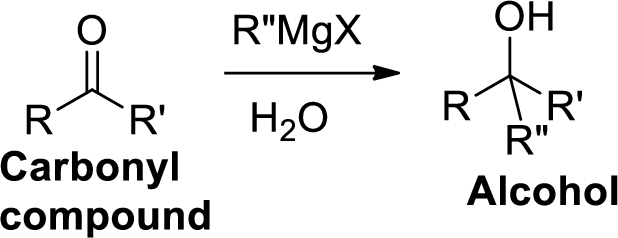
(b)
Interpretation:
The chirality of Carbinoxamine is to be identified and the possible stereoisomers has to be identified in the given synthesis.
Concept introduction:
Chiral:
A molecule is non superimposable on its mirror image is called chiral molecule.
Four different atoms attached to a carbon atom is called chiral molecule.
Isomer:
A molecule having the same molecular formula but with different chemical structure is called isomer.
Stereoisomers:
Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers:
A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers:
A compound which is non-superimposable and non-mirror image is called diastereomers.
Racemic mixture:
A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Organic Chemistry
- Calculate the reaction rate for selenious acid, H2SeO3, if 0.1150 M I-1 decreases to 0.0770 M in 12.0 minutes. H2SeO3(aq) + 6I-1(aq) + 4H+1(aq) ⟶ Se(s) + 2I3-1(aq) + 3H2O(l)arrow_forwardProblem 5-31 Which of the following objects are chiral? (a) A basketball (d) A golf club (b) A fork (c) A wine glass (e) A spiral staircase (f) A snowflake Problem 5-32 Which of the following compounds are chiral? Draw them, and label the chirality centers. (a) 2,4-Dimethylheptane (b) 5-Ethyl-3,3-dimethylheptane (c) cis-1,4-Dichlorocyclohexane Problem 5-33 Draw chiral molecules that meet the following descriptions: (a) A chloroalkane, C5H11Cl (c) An alkene, C6H12 (b) An alcohol, C6H140 (d) An alkane, C8H18 Problem 5-36 Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How H3C CH3 many chirality centers does erythronolide B have? OH Identify them. H3C -CH3 OH Erythronolide B H3C. H3C. OH OH CH3arrow_forwardPLEASE HELP! URGENT! PLEASE RESPOND!arrow_forward
- 2. Propose a mechanism for this reaction. ہلی سے ملی N H (excess)arrow_forwardSteps and explanationn please.arrow_forwardProblem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


