
Interpretation:
The IUPAC name of the following compound should be determined:
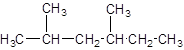
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be
Answer to Problem 8PP
2, 4-dimethylhexane.
Explanation of Solution
In order to give the IUPAC name to the alkane following steps are followed:
1. The parent (longest) continuous carbon chain is identified.
2. The ending of the parent chain for alkane (-e) is -ane.
3. Name should be written in alphabetical order and numbering should be done in such a way that the substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:

The given structure is:
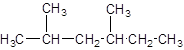
The parent chain contains 2 substituents so numbering is done in such a way that substituents gets lower number as shown:
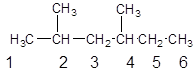
Since, the parent chain contains 6 carbon atoms and only single bonds are present so, the parent chain is hexane. Since, the substituents,
Interpretation:
The IUPAC name of the following compound should be determined:
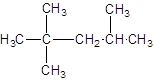
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 8PP
2, 2, 4-trimethylpentane.
Explanation of Solution
The given structure is:
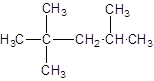
The parent chain contains 3 substituents so numbering is done in such a way that substituents gets lower number as shown:
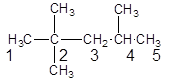
Since, the parent chain contains 5 carbon atoms and only single bonds are present so, the parent chain is pentane. Since, the substituents,
Interpretation:
The IUPAC name of the following compound should be determined:
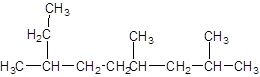
Concept introduction:
The hydrocarbon compounds that contains single bond(s) between carbon atom(s) are said to be saturated hydrocarbon. Hydrocarbon compounds containing single bonds only are said to be alkane.
Answer to Problem 8PP
2, 4, 7-trimethylnonane.
Explanation of Solution
The given structure is:
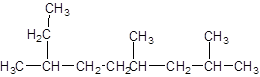
The parent chain contains 3 substituents so numbering is done in such a way that substituents gets lower number as shown:
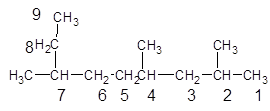
Since, the parent chain contains 9 carbon atoms and only single bonds are present so, the parent chain is nonane. Since, the substituents,
Chapter 21 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Chemistry: The Central Science (14th Edition)
Inorganic Chemistry
Organic Chemistry
Organic Chemistry (8th Edition)
CHEMISTRY-TEXT
Chemistry: A Molecular Approach (4th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





