
Interpretation:
The structure of 1-ethyl-3-propylcyclopentane needs to be drawn.
Concept introduction:
When the carbon atoms of hydrocarbons are arranged in such a way that it results in the formation of ring then it is said to be cycloalkanes.
Answer to Problem 11PP
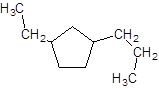
Explanation of Solution
The given name is 1-ethyl-3-propylcyclopentane that means the parent chain contains cyclic ring of 5 carbon atoms and -ethyl represents the presence of ethyl substituent at position 1 and -propyl substituent at position 3. So, the structure of 1-ethyl-3-propylcyclopentane is:
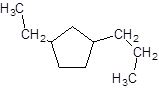
Interpretation:
The structure of 1, 2, 2, 4-tetramethylcyclohexane needs to be drawn.
Concept introduction:
When the carbon atoms of hydrocarbons are arranged in such a way that it results in the formation of ring then it is said to be cycloalkanes.
Answer to Problem 11PP
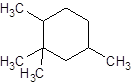
Explanation of Solution
The given name is 1, 2, 2, 4-tetramethylcyclohexane that means the parent chain contains cyclic ring of 6 carbon atoms and -tetramethyl represents the presence of four methyl substituent at position 1, two methyl at 2 and 4. So, the structure of 1, 2, 2, 4-tetramethylcyclohexane is:
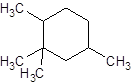
Chapter 21 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Campbell Essential Biology (7th Edition)
Chemistry: A Molecular Approach (4th Edition)
Microbiology: An Introduction
Introductory Chemistry (6th Edition)
Campbell Biology (11th Edition)
- ClONO2 is stable during the Antarctic winter, but quickly decomposes to form •ClO and •NO2 during the Antarctic summer. Explain why this is so.arrow_forwardThe EPA sets exposure standards for dangerous pollutants. What is the definition of a “24-hour standard.” What is the current 24-hour standard for PM2.5 (µg/m3)?arrow_forwardRubidium occurs naturally in two isotopes: 85Rb and 87Rb. Rubidium-85, with a relative abundance of 72%, has nuclear spin I = 5/2. The nuclear spin of 87Rb is I = 3/2. The ground state of the single valence electron in rubidium (atomic number 37) is 5s. The first excited state is the 5p. Rather than being single energy levels, the atomic energy levels are split into closely spaced sub-levels of energy EJ, where J = L + S is the total electronic angular momentum of the state. This energy splitting, designated fine structure splitting, originates from the spin-orbit coupling of the electron. The splitting between the ground and first excited state is 780.24 nm. The splitting between the ground state and the second excited state is 794.6 nm. The fine structure splitting is the same for both isotopes of rubidium. In addition to fine structure splitting, the energy levels are further separated into different F sub-levels, where F = I + J and I is the nuclear spin. Allowed F values range…arrow_forward
- Reactions involving ozone are largely responsible for the temperature layer of the stratosphere. Describe two ways that ozone contributes to warming of the stratosphere. You may wish to include chemical reactions.arrow_forwardOceans are the largest reservoir of carbon on the planet. Since 1751, average surface pH of the ocean has declined by 0.11 pH (some regions have seen local decline approaching a full unit pH). Consider the equilibrium CO2 dissolution reaction and the diagram above. How would you expect increased ocean acidity to impact the oceans’ capacity to dissolve CO2? Explain your answer.arrow_forward(a) Give a brief description of fluorescence and phosphorescence where you clearly state the fundamental difference between them. (b) Phosphorescence is the result of a forbidden transition. What does this mean? (c) Draw an energy level diagram showing the electronic states as Morse potentials that give rise to fluorescence and phosphorescence.arrow_forward
- 3. Consider the distribution diagram for inorganic C in water. Answer the following questions. 1.0 0.8 CO₂ (H2CO3) Proportion of total inorganic C 0.2 i2 90 0.4 90 0.6 0.0 HCO3 3 4 5 6 7 8 9 10 11 12 PH Carbon dioxide dissolves in water according to the equilibrium CO2(g) + H2O(1) D H2CO3(aq). a. The average pH of surface ocean water is 8.14. In what form(s) (carbonic acid, bicarbonate ion, or carbonate ion) does most dissolved CO2 occur in ocean water?arrow_forwardExplain the general change in •OH concentration over the course of a day. Why is it greatest at midday and lowest overnight? Please include one or more chemical reactions as part of your answer.arrow_forwardIn the absence of a catalyst, the rate constant for the overall reaction is on the order of k(298 K) ~ 10–26 M–1. The catalyzed reaction has a rate constant on the order of k(298 K) ~ 107 M–1 (step 1 is the rate determining step). Would you expect CO to be significantly oxidized in the absence of a catalyst? Is the uncatalyzed process thermodynamically limited, or kinetically limited? Explain your answer.arrow_forward
- Which of the following is predicted to be used by the sulfur atom in SO2 based on valence bond theory? ○ sp² O s²p ○ sp ○ sp³arrow_forwardDraw the conformed out of which an E2 elimination reaction can occur?arrow_forwardIn order for a molecule to absorb infrared radiation, the vibrational mode must exhibit a change in the dipole moment. HCI in the gas phase is used in Physical Chemistry Lab to demonstrate t vibrational spectroscopy. 1.40 1.20 1.00 0.80 0.60 0.40 0.20 0.00 2600 2650 2700 2750 2800 2850 2900 2950 3000 cm1 3050 3100 Figure 1: The infrared absorption spectrum of gas phase H35 Cl and H37 Cl. These data can be fit using a multiple least squares regression using the following equation where m is used to index each rotational peak in the spectrum. The frequency of each peak is given by (m) = vo+ (2Be-2αe)m - αem² - 4Dem³ (1) where vo is the frequency of the v=0, J" =0 → v=1, J'=0 forbidden transition (i.e. this is the "missing" Q branch), Be is the rotational constant relative to the equilibrium internuclear separation, de is the vibration-rotation coupling constant and De is the centrifugal distortion constant*. Note that high m transitions are the most important for determining De due to…arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





