
Concept explainers
a.
Interpretation:
The condensed structural formula of 1, 2-dimethylcyclopropane needs to be drawn.
Concept introduction:
When the carbon atoms of hydrocarbons are arranged in such a way that it results in the formation of ring then it is said to be cycloalkanes (containing single bond only in the ring).
a.
Answer to Problem 57A
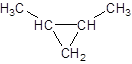
Explanation of Solution
The given name is 1, 2-dimethylcyclopropane that means the parent chain contains cyclic ring of 3 carbon atoms and -dimethyl represents the presence of two methyl substituents at position 1 and 2. So, the structure of 1, 2-dimethylcyclopropane is:
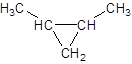
b.
Interpretation:
The condensed structural formula of 1, 1-diethyl-2-methylcyclopentane needs to be drawn.
Concept introduction:
When the carbon atoms of hydrocarbons are arranged in such a way that it results in the formation of ring then it is said to be cycloalkanes (containing single bond only in the ring).
b.
Answer to Problem 57A
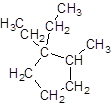
Explanation of Solution
The given name is 1, 1-diethyl-2-methylcyclopentane that means the parent chain contains cyclic ring of 5 carbon atoms and -diethyl represents the presence of two ethyl substituents at position 1 and one methyl substituent at position 2. So, the structure of 1, 1-diethyl-2-methylcyclopentane is:
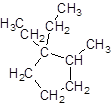
Chapter 21 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Chemistry: A Molecular Approach
Introductory Chemistry (5th Edition) (Standalone Book)
Essential Organic Chemistry (3rd Edition)
General Chemistry: Principles and Modern Applications (11th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry: The Central Science (13th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





