
Concept explainers
(a)
Interpretation: A cyclic compound that is an isomer of trans-2-butene needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(a)
Answer to Problem 49E

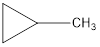
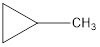
Explanation of Solution
The formula of trans-2-butane is C4H8. The cyclic compounds that is an isomer of trans-2-butene are as follows:

(b)
Interpretation: An ester that is an isomer of propanoic acid needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(b)
Answer to Problem 49E
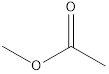
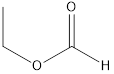
Methyl ethanoate ethyl methanoate
Explanation of Solution
The molecular formula of propanoic acid is
Esters which are isomers of propanoic acid are as follows:
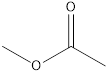
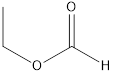
Methyl ethanoate ethyl methanoate
(c)
Interpretation: A
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(c)
Answer to Problem 49E
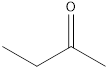
2-butanone
Explanation of Solution
The molecular formula of butanal is
A ketone that is an isomer of butanal is as follows:
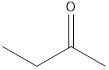
2-butanone
(d)
Interpretation: Secondary
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(d)
Answer to Problem 49E
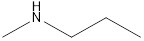

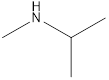
Methyl-propyl-amine Diethyl-amine Isopropyl-methyl-amine
Explanation of Solution
Secondary amine that is an isomer of butylamine are,
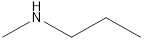

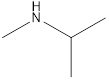
Methyl-propyl-amine Diethyl-amine Isopropyl-methyl-amine
(e)
Interpretation: A tertiary amine that is an isomer of butylamine needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(e)
Answer to Problem 49E
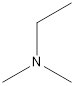
Ethyl-dimethyl-amine
Explanation of Solution
A tertiary amine that is an isomer of butylamine is as follows:
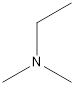
Ethyl-dimethyl-amine
(f)
Interpretation: An ether that is an isomer of 2-methyl-2-propanol needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(f)
Answer to Problem 49E
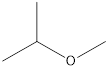


2-Methyoxy-propane Ethoxy-ethane 1-Methoxy-propane
Explanation of Solution
The formula of 2-methyl-2-propanol is
Ethers that are isomers of 2-methyl-2-propanol are as follows:
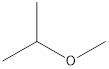


2-Methyoxy-propane Ethoxy-ethane 1-Methoxy-propane
(g)
Interpretation: A secondary alcohol that is an isomer of 2-methyl-2-propanol needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(g)
Answer to Problem 49E
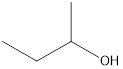
2-butanol
Explanation of Solution
The formula of 2-methyl-2-propanol is
Secondary alcohol that is an isomer of 2-methyl-2-propanol is as follows:
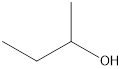
2-butanol
Want to see more full solutions like this?
Chapter 21 Solutions
Chemical Principles
- 2. Draw the structure for each compound and classify the amine as primary, secondary. or tertiary. a. 3-aminopentane b. 1,6-diaminohexane c. ethylphenylamine 3. Draw the structure for each compound. a. Aniline b. m-bromoanilinearrow_forwardIdentify the organic functional groups and reaction type for the following reaction.The reactant is a(n)a. secondary amideb. carboxylic acidc. tertiary amided. aromatice. ketonef. primary amideg. aldehydeh. amineThe products are a(n)a. carboxylate ion and amineb. ketone and aminec. carboxylic acid and amided. carboxylic acid and alcohole. ester and aminef. aldehyde and amineg. carboxylic acid and ammonium ionThe reaction type isa. hydrolysis (in acid)b. amide synthesisc. hydrationd. esterificatione. dehydrationf. hydrolysis (in base)arrow_forward1. what grouo does the ff organic compound belong? a. ketone b. ether c. cyloalkane d. esther 2. what group does the ff organic compound belong? a. amide b. azo c. nitrile d. amine 3. what is the priority functional group of the ff organic compound? a. carboxyl b. hydroxyl c. carbonyl d. hydroxidearrow_forward
- 1. The following molecule contains the functional groups A. secondary amine, secondary alcohol, ketone, carboxylic ester B. amide, primary alcohol, aldehyde, carboxylic acid C. secondary amine, two secondary alcohols, and two ketones D. primary amine, secondary alcohol, ketone, and carboxylic acid The following molecule contains the functional groups 2. A. primary alcohol and primary amine C. secondary alcohol and primary amine 3. A. alkene and secondary alcohol C. ketone and seconary alcohol The following molecule contains the functional groups B. ether and ketone D. ether and alkene 4. The following molecule A. primary amine and ketone C. secondary amine and alkyne NH₂ contains the functional groups B. secondary amine and alkene D. secondary amine and ketone ОН OH B. secondary alcohol and secondary amine D. primary alcohol and secondary amine NH₂ OHarrow_forwardRCOOH + Sncl2/NH3 = ---------------------. a. Cyanides b. Amine c. Amide d. Niltrilearrow_forwardMatch the following molecules with the name of their functional group. A. Amine B. Amide C. Alcohol D.Ether E. Aldehyde F. Ketone G.Carboxcylic acid E.Ester Thanks.arrow_forward
- Draw condensed and skeletal structures for each of the following amines a. 2-methyl-N-propyl-1-propanamine b. N-ethylethanamine c. 5-methyl-1-hexanamine d. methyldipropylaminee. e. N,N-dimethyl-3-pentanamine f. cyclohexylethylmethylaminearrow_forwardName each amine.arrow_forwardWhat is the full structure of an amine which is an isomer of ethylamine? What is the isomer called? Is it primary, secondary or tertiary?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


