
Concept explainers
(a)
Interpretation: The solubility of minoxidil in acidic and basic aqueous solution should be determined.
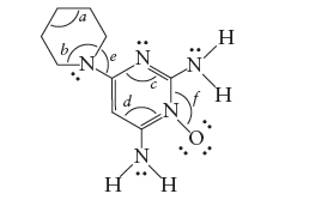
Concept Introduction: The Lewis structure of an organic compound represents the bonding of atoms with lone pairs (if any). It indicates the bonds with atoms and also arrangement of atoms in molecule.
Hybridization of any atom indicates the molecular geometry of molecule. The formula to check the hybridization can be written as:
Hybridization = Number of sigma bonds + Number of lone pair
(a)
Answer to Problem 40E
Minoxidil must be soluble in acidic aqueous solution.
Explanation of Solution
Minoxidil contains two −NH2 groups. The
As in acidic solution, it will form salt and therefore will be soluble.
(b)
Interpretation: The hybridization of five nitrogen atoms in minoxidil should be determined.
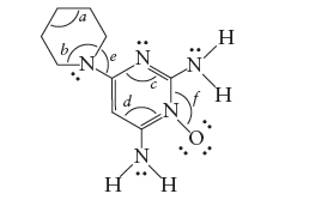
Concept Introduction: The Lewis structure of an organic compound represents the bonding of atoms with lone pairs (if any). It indicates the bonds with atoms and also arrangement of atoms in molecule.
Hybridization of any atom indicates the molecular geometry of molecule. The formula to check the hybridization can be written as:
Hybridization = Number of sigma bonds + Number of lone pair
(b)
Answer to Problem 40E
Explanation of Solution
Minoxidil contains two −NH2 groups. There are three N atoms in the ring. Thus the hybridization of N atoms are:
(c)
Interpretation: The hybridization of each carbon atoms in minoxidil should be determined.
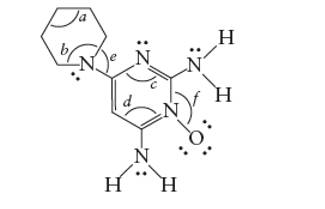
Concept Introduction: The Lewis structure of an organic compound represents the bonding of atoms with lone pairs (if any). It indicates the bonds with atoms and also arrangement of atoms in molecule.
Hybridization of any atom indicates the molecular geometry of molecule. The formula to check the hybridization can be written as:
Hybridization = Number of sigma bonds + Number of lone pair
(c)
Answer to Problem 40E
Explanation of Solution
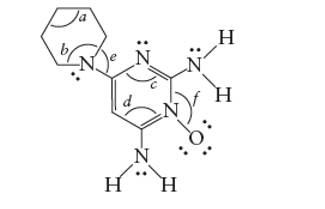
Minoxidil contains two types of C atoms. The double bonded carbon atoms are part of one ring whereas another ring contains only single bonded C atom.
(d)
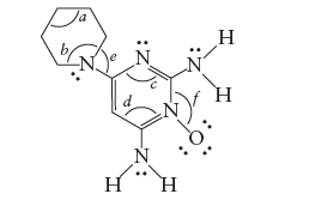
Interpretation: The bond angles marked as a, b, c, d, e and f in the given molecule of minoxidil should be determined.
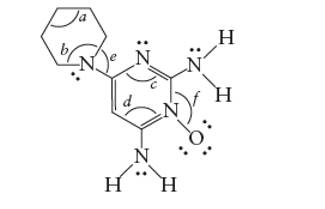
Concept Introduction: The Lewis structure of an organic compound represents the bonding of atoms with lone pairs (if any). It indicates the bonds with atoms and also arrangement of atoms in molecule.
Hybridization of any atom indicates the molecular geometry of molecule. The formula to check the hybridization can be written as:
Hybridization = Number of sigma bonds + Number of lone pair
(d)
Answer to Problem 40E
- 109°
- 109°
- 120°
- 120°
- 109°
- 120°
Explanation of Solution
The hybridization of bonded atoms determines the bond angle.
A sp3 hybrid C atom has 109° bond angle with tetrahedral geometry whereas a sp2 hybrid C atom has 120 ° bond angle with trigonal planer geometry. Similarly the single bonded N atom has 109° bond angle whereas a double bonded N atom has 120° bond angle. Thus, the marked bond angles must be:
- 109°
- 109°
- 120°
- 120°
- 109°
- 120°
Interpretation: Interpret the number of sigma bonds in the given molecule including hydrogen atoms.
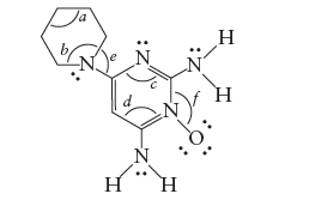
Concept Introduction: The Lewis structure of an organic compound represents the bonding of atoms with lone pairs (if any). It indicates the bonds with atoms and also arrangement of atoms in molecule.
Hybridization of any atom indicates the molecular geometry of molecule. The formula to check the hybridization can be written as:
Hybridization = Number of sigma bonds + Number of lone pair
Answer to Problem 40E
There are total 30 sigma bonds in the molecule.
Explanation of Solution
A single covalent bond is formed by one sigma bond whereas one double bond is composed of one sigma bond and one pi bond.
Each C atom must be bonded with four other atoms. Therefore in the bond line formula, each C atom must be bonded with other C or N atom and the tetravalency will be completed with H atoms.
Thus there are total 30 sigma bonds in the given molecule.
(f)
Interpretation: Interpret the number of p-bonds in the given molecule.
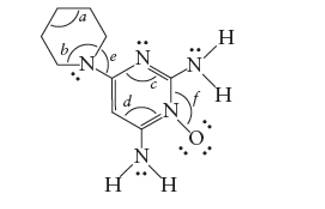
Concept Introduction: The Lewis structure of an organic compound represents the bonding of atoms with lone pairs (if any). It indicates the bonds with atoms and also arrangement of atoms in molecule.
Hybridization of any atom indicates the molecular geometry of molecule. The formula to check the hybridization can be written as:
Hybridization = Number of sigma bonds + Number of lone pair
(f)
Answer to Problem 40E
There are total 3 p-bonds in the molecule.
Explanation of Solution
A single covalent bond is formed by one sigma bond whereas one double bond is composed of one sigma bond and one pi bond.
Each C atom must be bonded with four other atoms. Therefore, in the bond line formula, each C atom must be bonded with other C or N atom and the tetravalency will be completed with H atoms.
Thus, there are total 3 p-bonds in the given molecule.
Want to see more full solutions like this?
Chapter 21 Solutions
Chemical Principles
- Identify any carbon atoms that change hybridization and the change in hybridization during the reactions in Exercise 20.51.arrow_forwardGamma hydroxybutyric acid, GHB, infamous as a date rape drug, is used illicitly because of its effects on the nervous system. The condensed molecular formula for GHB is HO(CH2)3COOH. (a) Write the Lewis structure for GHB. (b) Identify the hybridization of the carbon atom in the CH2 groups and of the terminal carbon. (c) Is hydrogen bonding possible in GHB? If so, write Lewis structures to illustrate the hydrogen bonding. (d) Which carbon atoms are involved in sigma bonds? In pi bonds? (e) Which oxygen atom is involved in sigma bonds? In pi bonds?arrow_forwarda. What is hybridization of carbon atom labeled "hybridization 3" b. Sigma (σ) bond between two carbon atoms (which DON'T have additional pi bond between them) is a combination of which two atomic orbitals according to the hybridization concept? c. Pi (π) bond between two carbon atoms is a combination of which two atomic orbitals according to the hybridization concept? d. Sigma (σ) bond between two carbon atoms (which are connected with pi bond as well) is a combination of which two atomic orbitals according to the hybridization concept? e. What is hybridization of oxygen atom labeled "hybridization 1" f. What is hybridization of carbon atom labeled "hybridization 2"arrow_forward
- why must an sp3 hybridized carbon with 4 different groups attached must be chiral?arrow_forwardAcetic acid can be made by the oxidation of acetaldehyde(CH3CHO). Molecules of acetaldehyde have a-CH3 group, an oxygen atom, and a hydrogen atom attached to a carbon atom. Draw the Lewis diagram for this molecule, give the hybridization of each carbon atom, and describethe p orbitals and the number of electrons that occupy each one. Draw the three-dimensional structure of the molecule, showing all angles.arrow_forward3 H. is C-C=N Nitrogen H H The hybridization of the red Nitrogen is H A/ A -C=N: H The geometry of the blue Nitrogen The hybridization of the bluearrow_forward
- 地 General formula of alkene - 5 .compounds are A - CnH2n+2. O B - CnH2n-2. O C - CnH2n. O The hybridization of carbon atom - 6 in alkane is - O A Sp^3 (30) + 1 n. B-Sp^3 (40) O С - Sp^3 (20) + 2 . Oarrow_forwardCHOOSE THE LETTER OF THE CORRECT ANSWER. What is the type of hybridization of the oxygen carbon in the organic molecule? Reaction mechanisms of organic molecules (alcohol to carboxylic acid) a. sp³ b. sp c. sp¹‧⁵ d. sp²arrow_forwardThe compound TCDD, or 2,3,7,8-tetrachlorodibenzo-p-dioxin gained a lot of public attention as a highly toxic compound in 2004 with it was used in the attempted murder of a Ukrainian politician. What structural characteristics of this compound give rise to it's toxicity? Select one or more: O a. The molecule is polar O b. The molecule is non-planar O c. The molecule is planar O d. The molecule is non-polararrow_forward
- 1:Y0 +AE A Oa"ll Asiacell امتحان الشهر الأول الوقت المتبقی 00:26:40 سؤال 8 1.00 الدرجة من Butadiene has-1,2 اخترأحد الخيارات a. only sp3 hybridized carbon atoms b. only sp2 hybridized carbon atoms c. only sp hybridized carbon atoms d. sp, sp2, sp3 hybridized carbon atoms أخل اختياري غير مجاب عليه بعد سؤال 9 الدرجة من 0 1.0 Which of the following alkanes will have the ?highest boiling point اخترأحد الخيارات Rutenearrow_forward5. Peptides and Proteins consist of amino acids linked together through amide bonds to form long polymers (below). a. What is the hybridization of the nitrogen atoms? Explain your answer b. What is the molecular geometry of the nitrogen atoms? c. All of the atoms in the box are in the same plane (this is an important structural aspect of proteins – it creates the necessary rigidity in the backbone for the protein's 3-D structure). Explain why they are all in the same plane. R Harrow_forward101. Hybridization of C2 and C3 ofarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax



