
Concept explainers
(a)
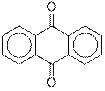
Interpretation: The given compound anthraquinone needs to be identified as an
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 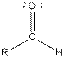 |
| Ketone | 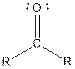 |
| Carboxylic acid | 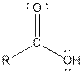 |
| Ester | 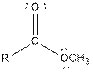 |
| Amine | 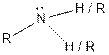 |
(a)
Answer to Problem 37E
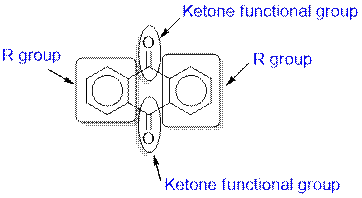
Anthraquinone contains a ketone functional group.
Explanation of Solution
From the table, the general structure for ketone is as follows:
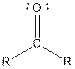
The given compound contains a ketone functional group shown as:
(b)
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
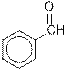
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 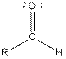 |
| Ketone | 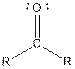 |
| Carboxylic acid | 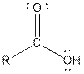 |
| Ester | 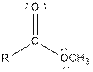 |
| Amine | 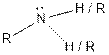 |
(b)
Answer to Problem 37E
The given organic compound contains an aldehyde functional group.
Explanation of Solution
From the table, the general structure for an aldehyde is as follows:
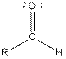
The given compound contains an aldehyde functional group shown as:

(c)
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
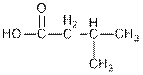
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 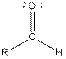 |
| Ketone | 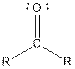 |
| Carboxylic acid | 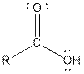 |
| Ester | 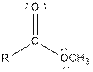 |
| Amine | 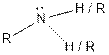 |
(c)
Answer to Problem 37E
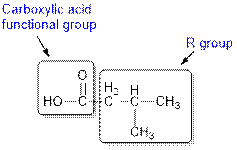
The given organic compound contains a carboxylic acid functional group.
Explanation of Solution
From the table, the general structure for carboxylic acid functional group is as follows:
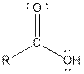
The given compound contains a carboxylic acid functional group shown as:
(d)
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
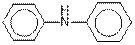
Concept Introduction: Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 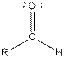 |
| Ketone | 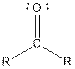 |
| Carboxylic acid | 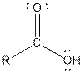 |
| Ester | 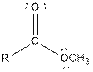 |
| Amine | 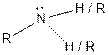 |
(d)
Answer to Problem 37E
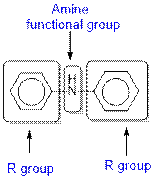
The given organic compound contains an amine functional group.
Explanation of Solution
From the table, the general structure for amine functional group is as follows:
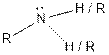
The given compound contains a carboxylic acid functional group shown as:
Want to see more full solutions like this?
Chapter 21 Solutions
Chemical Principles
- in medicine as the first line of defence against breast cancer. Identify ALL the different functional groups in the molecule. оно NH ÖH O A. Aromatic ring, carboxylic acid, ester, amide, alkene, alcohol, cyclic ether. B. Substituted benzene ring, amide, alcohol, ester, alkene, ketone, cyclic ether. C. Substituted benzene ring, carboxylic acid, ester, amide, alkene, alcohol, aldehyde. D. Aromatic ring, ketone, cyclic ester, amide, alkene, alcohol, aldehyde. E. Aromatic ring, aldehyde, ester, amide, alkene, alcohol, acyclic ether.arrow_forwardamine ester 49. The general structure of the sulfur containing compound R-C-O-SH is for O thiols thials O thio acids 50. What is the name of this compound 3HC-CH2-CH2-CH2-CH2-CH2- NH2?arrow_forwardWhat are the major products of the reaction of ethyl benzoate with hydrochloric acid and water? a. acetic acid and toluene b. phenylic acid and ethanol C. ethanoic acid and benzene d. benzoic acid and ethanol e. phenylic acid and methanol O a O barrow_forward
- What are the major products of the reaction of ethyl benzoate with hydrochloric acid and water? a. benzoic acid and ethanol b. phenylic acid and ethanol c. ethanoic acid and benzene d. acetic acid and toluene e. phenylic acid and methanolarrow_forwardEster compounds often have a sweet, pleasant odor. Many characteristic fruit scents are largely due to the natural presence of one or more ester compounds. As such, artificial scents for foods are often composed of complex mixtures of various esters. The exact identity and ratio of ingredients that compose a particular scent are closely guarded secrets in the food and fragrance industry. Suppose that you are a chemist working for a company that i creating a new line of air fresheners. The company is considering three scents: apple, pear, and pineapple. The project manager has asked you to prepare the ester compounds that are largely responsible for these scents. The structural formulas for these ester compounds are shown here: Alcohols for Air Freshener Project Molar mass Density Cost, per (g/mL) Reagent (g/mol) 1.00 L methanol 32.04 0.79 $46.20 ethanol 46.07 0.79 $112.00 1-propanol 60.10 0.80 $72.70 1-butanol 74.12 0.81 $72.60 Use the structural formulas of the alcohols and…arrow_forward1. The rank as one of the most useful classes of organic compounds because they often serve as starting materials for the preparation of numerous other familos. * O aromatic hydrocarbons O alkyl (aliphatic) halides O aliphatic hydrocarbons are the compounds containing in the molecule the carboxyl functional group attached to the hydrocarbon radical.* 2. O Ketones Carboxylic acids Estersarrow_forward
- Arrange the following amines in increasing boiling point. O₂N NH₂ NH₂ NH₂ B A O a. Carrow_forward1. Which of the following statements is true? I. Aldehydes and ketones both contain a hydroxyl group. II. The names for aldehydes and ketones are derived from the name of the longest carbon chain that contains the carbonyl group. III. The aldehyde and ketone with a molecular formula of C3H6O are constitutional isomers. IV. 2-Propanone is immiscible in water. A. I & II B. II & II C. I & III D. I & IV 2. Whicb of the following is the correct IUPAC name of the structure below?arrow_forwardTrue or False: Write True if the statement is correct and False if otherwise.i. Esters are formed by the reaction of a salt with an acid.ii. In such reactions, the —OR group from the alcohol replaces the —OH group in the carboxylic acid.iii. Esters are polar compounds, but they cannot form hydrogen bonds to each other.iv. Their boiling points are lower than those of alcohols and acids of similar molecular massv. Esters cannot be converted back to carboxylic acids and alcohols under either acidic or basicconditions.vi. Thioesters are sulfur-containing analogs of esters in which a —SR group has replaced the —COOHgroup.vii. Polyesters are polymers in which the monomers (diacids and dialcohols) are joined through esterlinkages.viii. An ester is named as an alkyl carboxylate.arrow_forward1. Describe the structure and properties of carboxylic acids and esters. 2. Name at least two (2) common carboxylic acids and esters.arrow_forwardWhich of the following nitrogen-containing compounds are not amides? Explain why. a. b. d. `N´ `N´ H Write the Lewis structure and the skeletal line structure for N-ethylbutanamide.arrow_forwardGive a brief Introduction to Organic Molecules and Functional Groups ?arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


