
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 21, Problem 46P
Interpretation Introduction
Interpretation:
The three-letter abbreviations for the amino acids formed by hydrolysis of the tripeptide depicted in ball-and −stick model needs to be determined.
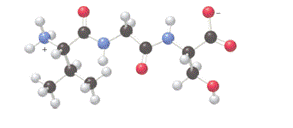
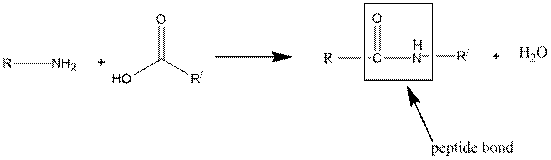
Concept introduction:
A peptide bond is a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The velocity distribution function of gas moleculesa) is used to measure their velocity, since the small size of gas molecules means that it cannot be measured in any other wayb) is only used to describe the velocity of particles if their density is very high.c) describes the probability that a gas particle has a velocity in a given interval of velocities
Explain why in the representation of a one-dimensional velocity distribution function for a particular gas, the maximum occurs for vi = 0 m/s.
Explain why the representation of a one-dimensional velocity distribution function for a particular gas becomes flatter as the temperature increases.
Chapter 21 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 21.2 - In addition to the amino and carboxyl groups, what...Ch. 21.2 - Draw both enantiomers of each amino acid in...Ch. 21.2 - Which of the following amino acids is naturally...Ch. 21.3 - Draw the structure of the amino acid valine at...Ch. 21.3 - Identify the amino acid shown with all uncharged...Ch. 21.4 - Identify the N-terminal and C-terminal amino acid...Ch. 21.4 - Prob. 21.4PCh. 21.4 - Prob. 21.4PPCh. 21.4 - Prob. 21.5PCh. 21.4 - Prob. 21.5PP
Ch. 21.4 - Prob. 21.6PPCh. 21.5 - Prob. 21.6PCh. 21.6 - Prob. 21.7PCh. 21.6 - Prob. 21.8PCh. 21.6 - Prob. 21.9PCh. 21.7 - Why is hemoglobin more water soluble than ...Ch. 21.8 - Prob. 21.7PPCh. 21.8 - Prob. 21.11PCh. 21.9 - Prob. 21.8PPCh. 21.9 - Prob. 21.12PCh. 21.9 - Prob. 21.9PPCh. 21.9 - Prob. 21.13PCh. 21.10 - Prob. 21.14PCh. 21.10 - Prob. 21.15PCh. 21.10 - Prob. 21.16PCh. 21.10 - Prob. 21.17PCh. 21.10 - The nerve gas sarin acts as a poison by covalently...Ch. 21.10 - Prob. 21.19PCh. 21.10 - Explain why the proteins involved in blood...Ch. 21 - The amino acid alanine is a solid at room...Ch. 21 - Why is phenylalanine water soluble but...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - Draw both enantiomers of each amino acid and label...Ch. 21 - Which of the following Fischer projections...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - (a) Identify the amino acid shown with all...Ch. 21 - Prob. 32PCh. 21 - Prob. 33PCh. 21 - Draw the structure of the neutral, positively...Ch. 21 - Locate the peptide bond in the dipeptide shown in...Ch. 21 - Label the N-terminal and C-terminal amino acids in...Ch. 21 - Melittin, the principal toxin of bee venom,...Ch. 21 - Cobratoxin is a neurotoxin found in the venom of...Ch. 21 - (a) Draw the structure of the two possible...Ch. 21 - (a) Draw the structure of the two possible...Ch. 21 - For each tripeptide: [1] draw the structure of the...Ch. 21 - For each tripeptide: [1] draw the structure of the...Ch. 21 - Prob. 43PCh. 21 - For each tripeptide: [1] identify the amino acids...Ch. 21 - What amino acids are formed by hydrolysis of the...Ch. 21 - Prob. 46PCh. 21 - Prob. 47PCh. 21 - Draw the structures of the amino acids formed when...Ch. 21 - Prob. 49PCh. 21 - Prob. 50PCh. 21 - Prob. 51PCh. 21 - Trypsin is a digestive enzyme that hydrolyzes...Ch. 21 - What type of intermolecular forces exist between...Ch. 21 - What type of interaction occur at each of the...Ch. 21 - Which peptide in each pair contains amino acids...Ch. 21 - Decide if the side chains of the amino acid...Ch. 21 - Which type of protein structure is indicated in...Ch. 21 - Label each of the following diagrams as...Ch. 21 - Prob. 59PCh. 21 - Prob. 60PCh. 21 - Prob. 61PCh. 21 - Prob. 62PCh. 21 - Compare - keratin and hemoglobin with regard to...Ch. 21 - Compare collagen and myoglobin with regard to each...Ch. 21 - Prob. 65PCh. 21 - Prob. 66PCh. 21 - Describe the function or biological activity of...Ch. 21 - Describe the function or biological activity of...Ch. 21 - Prob. 69PCh. 21 - Prob. 70PCh. 21 - What class of enzyme catalyzes each of the...Ch. 21 - What class of enzyme catalyzes each of the...Ch. 21 - Prob. 73PCh. 21 - Prob. 74PCh. 21 - Prob. 75PCh. 21 - What kind of reaction is catalyzed by each of the...Ch. 21 - Prob. 77PCh. 21 - How will each of the following changes affect the...Ch. 21 - Prob. 79PCh. 21 - Prob. 80PCh. 21 - Prob. 81PCh. 21 - Prob. 82PCh. 21 - Prob. 83PCh. 21 - Prob. 84PCh. 21 - Prob. 85PCh. 21 - Prob. 86PCh. 21 - Why must vegetarian diets be carefully balanced?Ch. 21 - Prob. 88PCh. 21 - Sometimes an incision is cauterized (burned) to...Ch. 21 - Why is insulin administered by injection instead...Ch. 21 - Prob. 91PCh. 21 - The silk produced by a silkworm is a protein with...Ch. 21 - Explain the difference in the mechanism of action...Ch. 21 - Prob. 94PCh. 21 - Prob. 95CPCh. 21 - Suggest a reason for the following observation....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a Lewis structure for each of the following molecules and assign charges where appropriate. The order in which the atoms are connected is given in parentheses. a. CIFCIF b. BrCNBrCN 0 c. SOCI2 × (CISCIO) SOC₁₂ (CISCI) You can draw both an octet and a valence shell expanded structure. Considering the following structural information, which is the better one: The measured S-OS-O bond length in SOC12SOCl2 is 1.43 Å. For comparison, that in SO2SO2 is 1.43 Å [Exercise 1-9, part (b)], that in CHзSOHCH3 SOH d. CH3NH2CH3NH2 (methanesulfenic acid) is 1.66 A. e. CH3OCH3 CH3 OCH3 NH2 f. N2H2× (HNNH) N2 H2 (HNNH) g. CH2COCH₂ CO h. HN3× (HNNN) HN3 (HNNN) i. N20 × (NNO) N2O (NNO)arrow_forwardbre The reaction sequence shown in Scheme 5 demonstrates the synthesis of a substituted benzene derivative Q. wolsd works 2 NH2 NaNO2, HCI (apexe) 13× (1 HNO3, H2SO4 C6H5CIN2 0°C HOTE CHINO₂ N O *O₂H ( PO Q Я Scheme 5 2 bag abouoqmics to sounde odi WEIC (i) Draw the structure of intermediate O. [2 marks] to noitsmot od: tot meinedogm, noit so oft listsb ni zaupaib bas wa (ii) Draw the mechanism for the transformation of aniline N to intermediate O. Spoilage (b) [6 marks] (iii) Identify the reagent X used to convert compound O to the iodinated compound [tom E P. vueimado oilovonsa ni moitos nolisbnolov ayd toes ai tedw nisiqx (iv) Identify the possible structures of compound Q. [2 marks] [2 marks] [shom 2] (v) bus noires goiribbeolovo xnivollot adj to subora sidab Draw the mechanism for the transformation of intermediate P to compound Q. [5 marks] vi (vi) Account for the regiochemical outcome observed in the reaction forming compound Q. [3 marks]arrow_forwardPROBLEM 4 Solved Show how 1-butanol can be converted into the following compounds: a. PROBLEM 5+ b. d. -C= Narrow_forward
- The vibrational contribution isa) temperature independent for internal energy and heat capacityb) temperature dependent for internal energy and heat capacityc) temperature independent for heat capacityd) temperature independent for internal energyarrow_forwardQuantum mechanics. Explain the basis of approximating the summation to an integral in translational motion.arrow_forwardQuantum mechanics. In translational motion, the summation is replaced by an integral when evaluating the partition function. This is correct becausea) the spacing of the translational energy levels is very small compared to the product kTb) the spacing of the translational energy levels is comparable to the product kTc) the spacing of the translational energy levels is very large compared to the product kTarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY