
Concept explainers
For each amino acid: [1] draw the L enantiomer in a Fischer projection; [2] classify the amino acid as neutral, acidic, or basic; [3] give the three-letter symbol; [4] give the one-letter symbol.
- leucine
- tryptophan
- lysine
- aspartic acid
(a)
Interpretation:
The L-isomer of leucine in Fisher projection needs to be drawn and should be identified as acidic, basic or neutral. The three letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
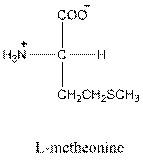
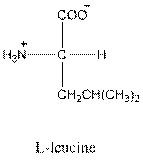
Answer to Problem 25P
- L-isomer of leucine is as follows:
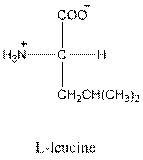
- Leucine is neutral.
- Three-letter symbol for leucine is Leu.
- One-letter symbol for leucine is L.
Explanation of Solution
For leucine the R group is
The amino acid leucine has one positive charge on amino group
One letter symbol for leucine is L and three-letter symbol for leucine is Leu.
(b)
Interpretation:
To draw L-isomer of tryptophan (amino acid) in Fisher projection, to identify it is acidic, basic or neutral and to give three letter and one symbol for it
Concept Introduction : :
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
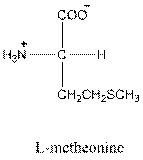
Answer to Problem 25P
- L-isomer of tryptophan is as follows:
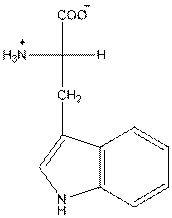
- Amino acid tryptophan is neutral.
Explanation of Solution
For tryptophan the R group is as follows:
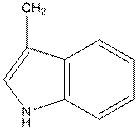
And in L-isomer the amino group
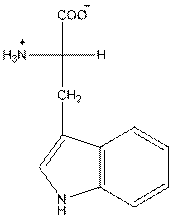
The amino acid tryptophan has one positive charge on amino group
One letter symbol for tryptophan is W and three-letter symbol for tryptophan is Trp.
(c)
Interpretation:
The L-isomer of lysine in Fisher projection needs to be drawn, whether it is acidic, basic or neutral needs to be determined and the three-letter and one symbol for it needs to be given.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 25P
- L-isomer of lysine is as follows:
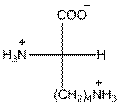
- The amino acid lysine is basic in nature.
- The three letter symbol for lysine is Lys.
- The one letter symbol for lysine is K.
Explanation of Solution
For lysine the R group is
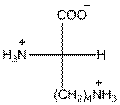
The amino acid lysine has one positive charge on amino group
The three letter symbol for lysine is Lys and one letter symbol for lysine is K.
(d)
Interpretation:
The L-isomer of aspartic acid in Fisher projection needs to be drawn, whether it is acidic, basic or neutral needs to be determined and the three-letter and one symbol for it needs to be given.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 25P
- L-isomer of aspartic acid is as follows:
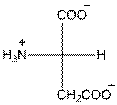
- The aspartic acid is acidic in nature.
- The three letter symbol for aspartic acid is Asp.
- The one letter symbol for aspartic acid is D.
Explanation of Solution
For aspartic acid the R group is
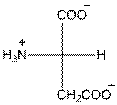
The aspartic acid has one positive charge on amino group
The three letter symbol for aspartic acid is Asp and the one letter symbol for aspartic acid is D.
Want to see more full solutions like this?
Chapter 21 Solutions
General, Organic, and Biological Chemistry - 4th edition
- 5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forwardShow work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. cleavage Bond A •CH3 + 26.← Cleavage 2°C. + Bond C +3°C• CH3 2C Cleavage E 2°C. 26. weakest bond Intact molecule Strongest 3°C 20. Gund Largest argest a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. C Weakest bond A Produces Most Bond Strongest Bond Strongest Gund produces least stable radicals Weakest Stable radical b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 13°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. • CH3 methyl radical Formed in Gund A Cleavage c.…arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





