
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 21.5E
Figure 21.35 shows a unit cell of diamond. Identify the atoms that define the unit cell and determine the Bravais lattice of this structure of diamond. How many atoms are in the unit cell?
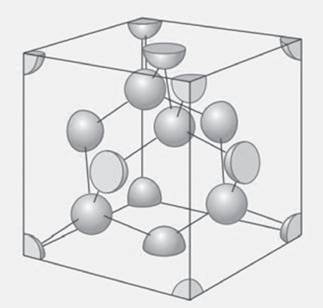
Figure 21.35 The unit cell of diamond. See exercise 21.5.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution,
respectively.
F CI
Br |
Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to
have a reasonable yield of product.
NH2
Br
Br
Br
OH
Br
Q7: Rank the following groups in order of basicity, nucleophilicity, and leaving group ability.
a) H₂O, OH, CH3COOT
b) NH3, H₂O, H₂S
Q8: Rank the following compounds in order of increasing reactivity in a nucleophilic substitution
reaction with CN as the nucleophile.
Br
A
B
NH2
LL
F
C
D
OH
CI
LLI
E
Q9: Complete the missing entities for following reactions (e.g., major product(s), reactants,
and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for
reactions a) to d).
a)
H
"Cl
D
+
-OCH 3
Page 3 of 5
Chapter 21 Solutions
Physical Chemistry
Ch. 21 - Prob. 21.1ECh. 21 - Boron nitride, BN, is a very hard material, harder...Ch. 21 - Prob. 21.3ECh. 21 - Prob. 21.4ECh. 21 - Figure 21.35 shows a unit cell of diamond....Ch. 21 - Prob. 21.6ECh. 21 - How many different unit cells can a crystal have...Ch. 21 - Prob. 21.8ECh. 21 - Prob. 21.9ECh. 21 - Prob. 21.10E
Ch. 21 - Prob. 21.11ECh. 21 - Prob. 21.12ECh. 21 - Prob. 21.13ECh. 21 - Prob. 21.14ECh. 21 - Prob. 21.15ECh. 21 - Prob. 21.16ECh. 21 - Prob. 21.17ECh. 21 - Prob. 21.18ECh. 21 - Prob. 21.19ECh. 21 - Prob. 21.20ECh. 21 - Prob. 21.21ECh. 21 - Prob. 21.22ECh. 21 - Prob. 21.23ECh. 21 - Prob. 21.24ECh. 21 - Prob. 21.25ECh. 21 - Prob. 21.26ECh. 21 - Prob. 21.27ECh. 21 - Prob. 21.28ECh. 21 - For a simple cubic lattice, what is the ratio of...Ch. 21 - Prob. 21.30ECh. 21 - Prob. 21.31ECh. 21 - Consider Figure 21.21. If the lower rightmost...Ch. 21 - Prob. 21.33ECh. 21 - The aluminum-nickel alloy AlNi has a simple cubic...Ch. 21 - Prob. 21.35ECh. 21 - The first two signals from a powdered sample has X...Ch. 21 - Prob. 21.37ECh. 21 - Prob. 21.38ECh. 21 - Prob. 21.39ECh. 21 - Prob. 21.40ECh. 21 - Prob. 21.41ECh. 21 - Prob. 21.42ECh. 21 - Prob. 21.43ECh. 21 - Prob. 21.44ECh. 21 - Prob. 21.45ECh. 21 - What is the coordination number in the cesium...Ch. 21 - Prob. 21.47ECh. 21 - Which solid phase that is, which allotrope of...Ch. 21 - Prob. 21.49ECh. 21 - Prob. 21.50ECh. 21 - Write Born-Haber cycles showing the relationship...Ch. 21 - Prob. 21.52ECh. 21 - Prob. 21.53ECh. 21 - Prob. 21.54ECh. 21 - The lattice energy for potassium iodide, KI, is...Ch. 21 - Prob. 21.56ECh. 21 - Prob. 21.57ECh. 21 - Prob. 21.58ECh. 21 - Prob. 21.59ECh. 21 - Prob. 21.60ECh. 21 - Prob. 21.61ECh. 21 - Prob. 21.62ECh. 21 - Prob. 21.63ECh. 21 - Prob. 21.64E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
- Explain why only the lone pairs on the central atom are taken into consideration when predicting molecular shapearrow_forward(ME EX1) Prblm #9/10 Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.arrow_forwardProblems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY