
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 20, Problem 20.56P
Write the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to “navigate” between the different
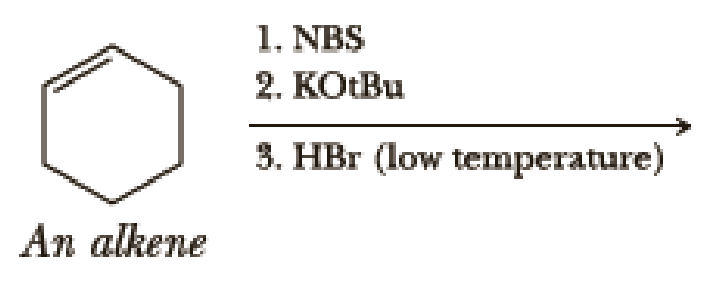
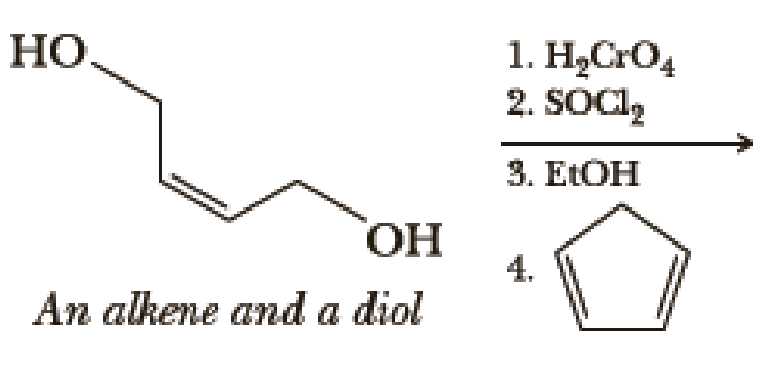
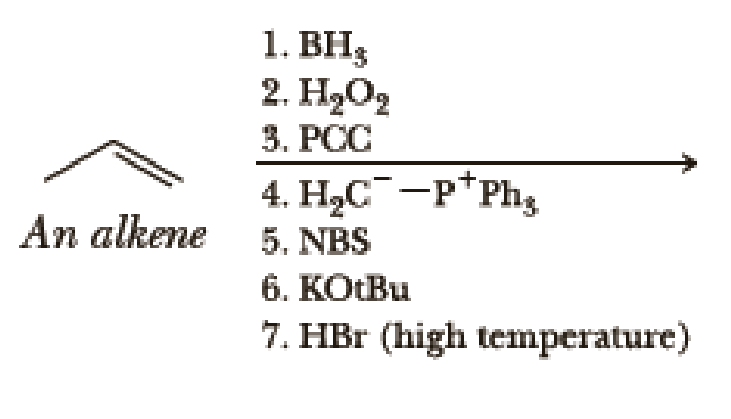
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please help me calculate the undiluted samples ppm concentration.
My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve.
Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4
Provide an IUPAC name for each of the compounds shown.
(Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to
commas, dashes, etc.)
H₁₂C
C(CH3)3
C=C
H3C
CH3
CH3CH2CH
CI
CH3
Submit Answer
Retry Entire Group
2 more group attempts remaining
Previous
Next
Arrange the following compounds / ions in increasing nucleophilicity (least to
most nucleophilic)
CH3NH2
CH3C=C:
CH3COO
1
2
3
5
Multiple Choice 1 point
1, 2, 3
2, 1, 3
3, 1, 2
2, 3, 1
The other answers are not correct
0000
Chapter 20 Solutions
Organic Chemistry
Ch. 20.1 - Prob. 20.1PCh. 20.1 - Estimate the stabilization gained as a result of...Ch. 20.2 - Predict the product(s) formed by addition of one...Ch. 20.3 - Prob. 20.4PCh. 20.3 - Prob. 20.5PCh. 20.4 - Prob. 20.6PCh. 20.5 - Prob. 20.7PCh. 20.5 - Prob. 20.8PCh. 20.5 - Prob. 20.9PCh. 20.6 - Prob. 20.10P
Ch. 20.6 - Prob. 20.11PCh. 20.6 - Prob. 20.12PCh. 20 - If an electron is added to 1,3-butadiene, into...Ch. 20 - Prob. 20.15PCh. 20 - Predict the structure of the major product formed...Ch. 20 - Predict the major product formed by 1,4-addition...Ch. 20 - Predict the structure of the major 1,2-addition...Ch. 20 - Prob. 20.19PCh. 20 - Prob. 20.20PCh. 20 - Prob. 20.21PCh. 20 - Prob. 20.22PCh. 20 - Prob. 20.23PCh. 20 - Pyridine exhibits a UV transition of the type n at...Ch. 20 - Prob. 20.25PCh. 20 - Prob. 20.26PCh. 20 - Prob. 20.27PCh. 20 - Write the frontier molecular orbital analysis for...Ch. 20 - Prob. 20.29PCh. 20 - Draw structural formulas for the products of...Ch. 20 - Propose structural formulas for compounds A and B...Ch. 20 - Under certain conditions, 1,3-butadiene can...Ch. 20 - Prob. 20.33PCh. 20 - Prob. 20.34PCh. 20 - The following triene undergoes an intramolecular...Ch. 20 - Prob. 20.36PCh. 20 - Prob. 20.37PCh. 20 - Prob. 20.38PCh. 20 - Prob. 20.39PCh. 20 - The Diels-Alder reaction is not limited to making...Ch. 20 - The first step in a synthesis of dodecahedrane...Ch. 20 - Bicyclo-2,5-heptadiene can be prepared in two...Ch. 20 - Prob. 20.43PCh. 20 - Prob. 20.44PCh. 20 - Following is a retrosynthetic scheme for the...Ch. 20 - Prob. 20.46PCh. 20 - Prob. 20.47PCh. 20 - Prob. 20.48PCh. 20 - Prob. 20.49PCh. 20 - Prob. 20.50PCh. 20 - What reaction presented in this chapter is...Ch. 20 - Claisen rearrangement of an allyl phenyl ether...Ch. 20 - Prob. 20.53PCh. 20 - Prob. 20.54PCh. 20 - We now continue the use of organic chemistry...Ch. 20 - Write the products of the following sequences of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forward
- Shown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forward
- Draw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M HCl is titrated with 37.75 mL of NaOH. What is the molarity of the NaOH?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY