
(a)
Interpretation: For the chair-like transition state of a
Concept Introduction:
It is a special class of pericyclic reaction in which a
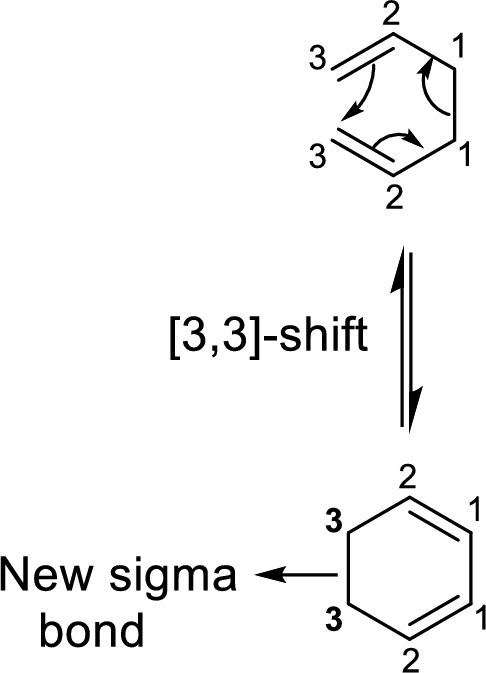
Stereochemistry in
Two types of transition states are likely to occur in this shift such as chair-like and boat-like transition states. According to the Frontier-Molecular-Orbital theory, both the transition states are allowed transition states inspite of the stability difference. The product obtained from the chair-like transition state has trans-conformation whereas the product obtained from the boat-like transition state has cis-conformation.
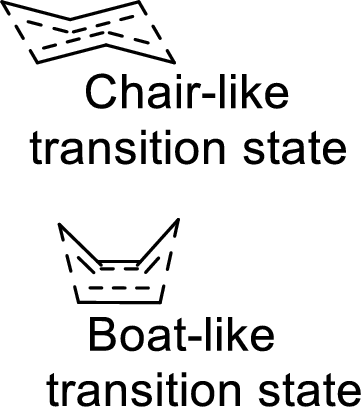
(b)
Interpretation: The reason for why the products with boat-like conformation are formed to a lower extent than those with chair-like conformation.
Concept Introduction:
It is a special class of pericyclic reaction in which a
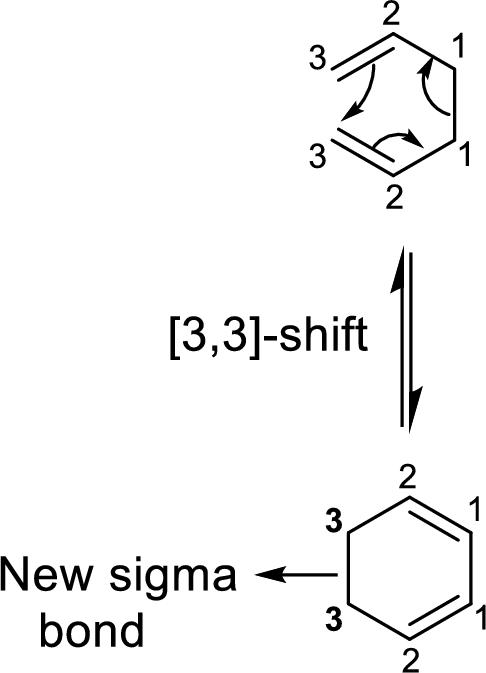
Stereochemistry in
Two types of transition states are likely to occur in this shift such as chair-like and boat-like transition states. According to the Frontier-Molecular-Orbital theory, both the transition states are allowed transition states inspite of the stability difference. The product obtained from the chair-like transition state has trans-conformation whereas the product obtained from the boat-like transition state has cis-conformation.
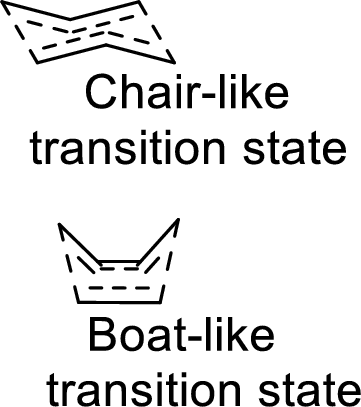
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry
- • 1. The HCo(CO)4 complex can catalyze the isomerization of pure cis-2-butene to a mixture of cis-2-butene, trans-2-butene, and 1-butene. Propose a mechanism. Carefully show the process using wedges and dashes to indicate stereochemistry of each intermediate.arrow_forwardC(CH3)2OH C(CH3):X +H20 where (X=F, CI, Br, I) give reaction with each of halogen as well determine with which halogen substitution reaction is more favorable and feasible and give reason of its feasibility?arrow_forward(a) Compound D undergoes a reaction with hydrogen bromide, HBr to produce 2-bromobutane. D exists as cis-trans isomers and decolourises bromine solution in methylene chloride, CH2CI2. Sebatian D mengalami tindak balas dengan hidrogen bromida, HBr untuk menghasilkan 2-bromobutana. D wujud sebagai isomer cis-trans dan memudarkan larutan bromin dalam metilena klorida, CH2CI2. (i) Draw and name the structure of compound D. Lukis dan namakan struktur sebatian D. (ii) Draw two (2) constitutional isomers of compound D. Lukis dua (2) isomer berjuzuk bagi sebatian D.arrow_forward
- Consider a hypothetical chemical reaction between compound A and compound B, which produces compound C as the final product. The reaction is known to be exothermic and spontaneous. However, when the reaction is carried out under certain conditions, it fails to occur. Explain this observation and propose a potential solution to overcome this hurdle.arrow_forward7. Draw the following ions. (a) cis- [PF.CI.) (b) trans- [PF.CI.J (c) fac- [PF.CIJ ( (d) Predict the P NMR spectra of a and c. From your 8. Draw Molecular orbital diagrams for O, 0, (superoxide) and O (peroxide) diagrams predict which, if any are paramagnetic? 구는 구 Paramagnetir Paramagnetic 구글arrow_forwardNaphthalene is a colorless solid with a dipole moment of zero. Azulene is a solid with an intense blue color and a dipole moment of 1.0 D. Account for the difference in dipole moments of these constitutional isomers.arrow_forward
- Starting with [Pt(NH3)4]2+ or [PtCl4]2- and using the trans effect sequence, devise a rational procedure for synthesizing [Pt(py)(NH3)(NO2)Cl] with py and Cl positioned trans to one another.arrow_forward(a) The Friedel-Crafts reaction of benzene with 2-chloro-3-methylbutane in the presence of AlCl3 occurs with a carbocation rearrangement. Give mechanistic explanation and the product formed. (b) Predict the product(s) will be formed from the following reactions: (i) Bromination of p-methylbenzoic acid (ii) Sulphonation of m-bromoanisole (iii) Friedel-craft acylation of o-bromonitrobenzenearrow_forwardThe first observation that this reaction is not a typical ligand substitution was the dependence on the pKb of the incoming ligand (using alkoxides) on the rate of substitution. It has been hypothesized that this is a base-hydrolysis mechanism. The key steps are shown below. [CoCl(NH3)5]2+ + OH– → [CoCl(NH2)(NH3)4]+ (1) [CoCl(NH2)(NH3)4]+ → [Co(NH2)(NH3)4]2+ + Cl– (2) [Co(NH2)(NH3)4]2+ + H2O → [Co(OH)(NH3)5]2+ + NH3 (3) (2) Our standard rate laws for associative and dissociative ligand substitution are rate = k[ML6][L’] and rate = k[ML6], respectively. Derive a rate law for the base-hydrolysis ligand substitution above where equation 1 is the rate determining step.arrow_forward
- A) Provide the reagent and reaction mechanism to show how the reactants and products in the following reaction can interconvert B) under what conditions would the reaction I) favour reactants, II) favour the products and III) why?arrow_forward1)Chemistry students are taking an experimental course in organic chemistry at a public university. During an experiment involving conjugated dienes, some doubts arose when discussing the results obtained so far: (a) A student obtained two products from the reaction of 1,3-cyclohexadiene with Br2. His lab partner was surprised to get only one product from the reaction of 1,3 - cyclohexadiene with HBr. Explain these distinct results. (b) One student, seeing the discussion of colleagues, commented that she obtained two distinct products when reacting 1,3,5-hexatriene with HBr, with different yields just by changing the reaction temperature. Explain the results she obtained using reaction mechanism and based on kinetic and thermodynamic control involving conjugated dienes.arrow_forwardb) Optical isomers can often be formed during these reactions. For products A-H indicate how many different optical isomers you expect to form for each from these reactions (you do not have to indicate whether they are diastereomers/enantiomers). c) In the preparation of substance D by ozonolysis, the second step is necessary treatment of the reaction mixture with zinc in acetic acid. If we forgot this step, we would get substance V instead of substance D, which would probably explode when isolated. What is the structure of substance V? *24 X 1: ozone V e) What products do you expect to form if they react with alkene X: I - HBr in diethyl ether; II - Brl in methanol; III - PhSCI in diethyl ether; IV - Selectfluor in methanol. P M EtOH NA EtONa PhSNa EtOH E H+ O TsCl Pyridín F- T2, Pd/C G4 mCPBA H₂SO4 10 %, H₂O 1: BH3 H◄ 2: H₂O₂ ill X Br₂ cyclohexane HCI A > В B HBr warmth, benzoylperoxid 1: ozone 2: Zn, ACOH Mg THF с D D₂O K I + J NaOHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





