
Concept explainers
Interpretation:
For the given reaction, the reverse Diels-Alder reaction path has to be shown with Lewis structures of Butadiene sulfone and sulfuric acid.
Concept Introduction:
Diels-Alder reaction:
It is the reaction of conjugated dienes with double or triple bonded compounds which are known as “dienophiles”. The reaction is a
Example:
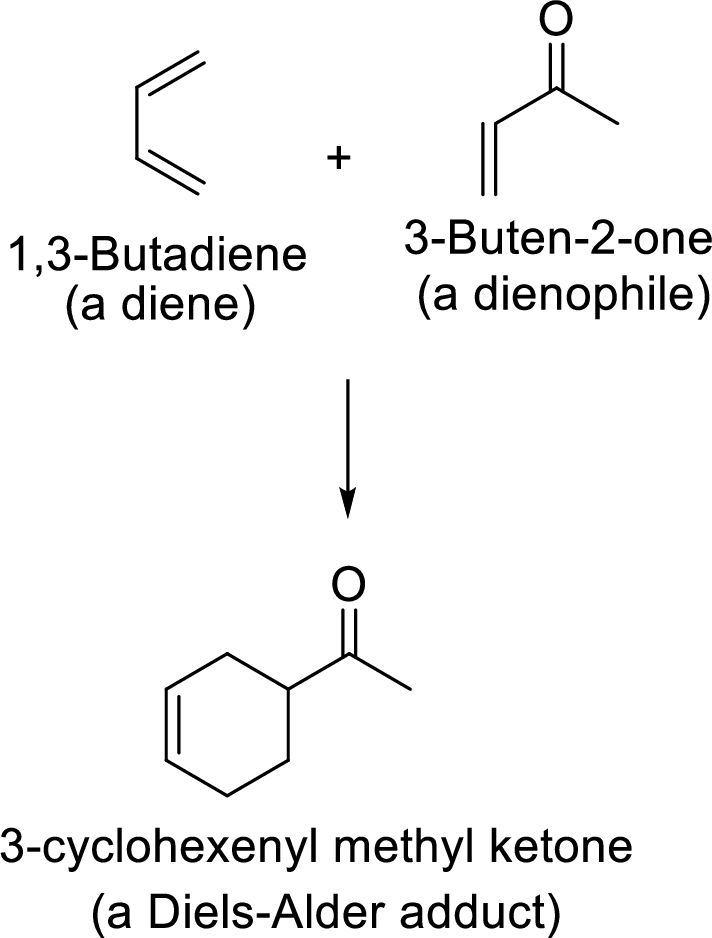
Reverse Diels-Alder reaction:
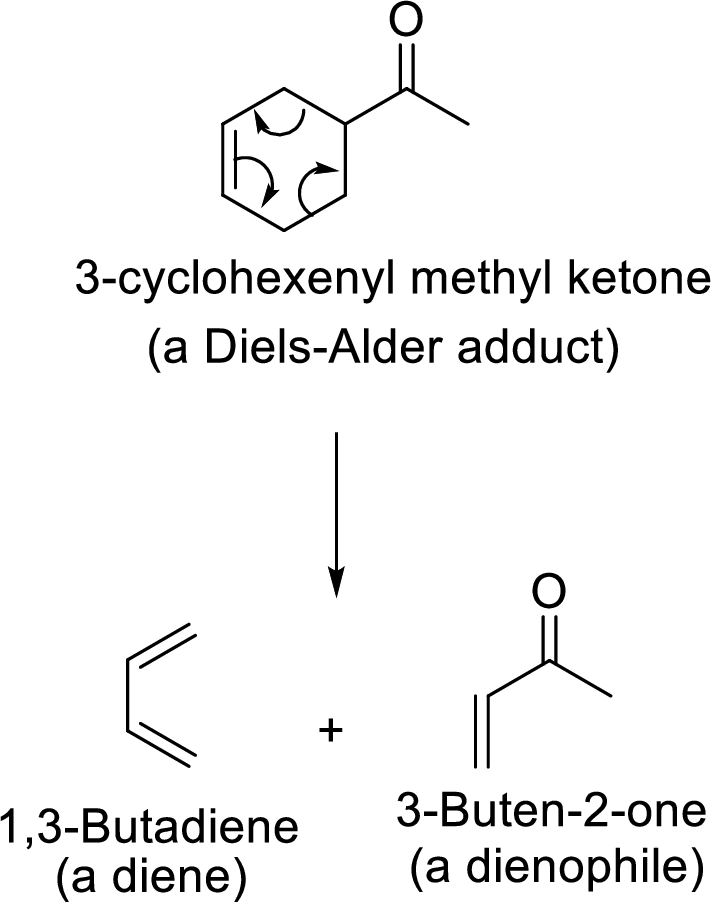
It is the reverse path of the Diels-Alder reaction in which the Diels-Alder adduct gives back its corresponding Diene and Dienophile as shown here:
Lewis dot structure: In the Lewis dot structure, an element will be represented by its elemental symbol. It will be surrounded by dots. Each dot represents the valence electron of that element. So the Lewis dot structure enables to represent an element with its valence electrons in
Trending nowThis is a popular solution!

Chapter 20 Solutions
Organic Chemistry
- The following triene undergoes an intramolecular Diels-Alder reaction to give a bicyclic product. Propose a structural formula for the product. Account for the observation that the Diels-Alder reaction given in this problem takes place under milder conditions (at lower temperature) than the analogous Diels-Alder reaction shown in Problem 20.34.arrow_forwardA chemist is attempting to synthesize a complex natural product with a highly strained cyclohexene ring system. Which type of reactants would be most suitable for achieving this goal, and why? Provide a detailed explanation of the choice of reactants and the expected outcome in terms of the Diels-Alder reaction.arrow_forwardWhich constitutional isomer represents the product of this Diels-Alder reaction?arrow_forward
- The Diels-Alder reaction is not limited to making six-membered rings with only carbon atoms. Predict the products of the following reaction that produce rings with atoms other than carbon in them.arrow_forwardMechanism The Diels-Alder reaction is part of a class of reactions known as a cycloaddition reaction. This reaction is specifically a [4+2] cycloaddition which is a concerted (one-step) process in which two new carbon - carbon sigma bonds are formed from two pi bonds. For the first Diels - Alder step of the mechanism fill in the arrows needed for the transformation. The rest of the mechanism is drawn for you. OH Show mechanism arrows for this step! 4 + 2 cycloaddition OH H D- & H+ transfer OH Nuc acyl substitution H L.G.arrow_forward7. At room temperature cyclopentadiene reacts with itself to form dicyclopentadiene in a Diels-Alder reaction. a) draw the self reaction of cyclopentadiene 20°C. b) When dicyclopentadiene is heated to boiling (170°C), the retor-Diels-Alder reaction occurs producing 2 moles of cyclopentadiene. Explain this observation in terms of free energy, enthalpy and entropy. c) If the AHxn = -75 kJ/mol and the ASpxn = -226 J/mol K, What temperature would be required for the reaction to be at equilibrium (Keq = 1).arrow_forward
- Choose the statement that is false regarding Diels-Alder reactions. A) The Diels-Alder reaction is concerted. B) The Diels-Alder reaction forms six-membered rings. C) The Diels-Alder reaction is stereospecific. D) Overall, two new C-C single bonds are formed and two C=C double bonds disappear. E) The product is favored in bicyclic Diels-Alder products.arrow_forwardPlease show all arrow pushingj mechaaisais. Thank you!arrow_forwardStep 6: Now that you have determined the substrates and mechanism of a Diels-Alder reaction, you will learn how to recognize when you should use the Diels-Alder reaction. In a synthesis reaction, if you are given a cyclohexene product with no other obvious functional group transformations and an electron-withdrawing group two carbons away from the alkene, it is likely made via the Diels-Alder reaction. Deduce the structures of the starting materials to form the Diels-Alder adduct shown. ..... CN CN Diene + Dienophilearrow_forward
- The following triene undergoes an intramolecular Diels-Alder reaction to give a bicyclic product. Propose a structural formula for the product. Account for the observation that the Diels-Alder reaction given in this problem takes place under milder conditions (at lower temperature) than the analogous Diels-Alder reaction 0"C Diels-Alder adductarrow_forwardCan someone draw the arrows for this reaction so I can understand how the product was formed? I'm having trouble understanding that aspect of the Diels-Alder reaction.arrow_forwardDiels—Alder reaction of a monosubstituted diene (such as CH2 = CH – CH = CHOCH3) with a monosubstituted dienophile (such as CH2 = CHCHO) gives a mixture of products, but the 1,2-disubstituted product often predominates. Draw the resonance hybrid for each reactant and use the charge distribution of the hybrids to explain why the 1,2-disubstituted product is the major product.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

