
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.22UKC
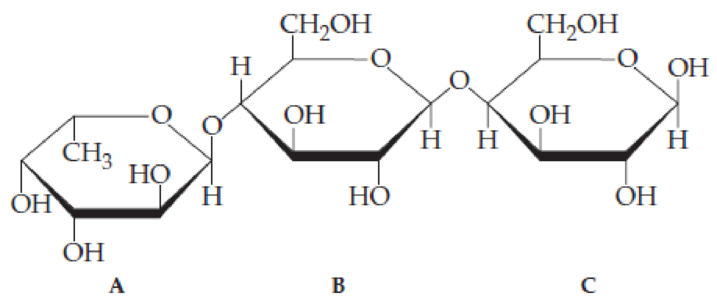
Consider the trisaccharide A, B, C shown in Problem 20.23.
- (a) Identify the hemiacetal and acetal linkages.
- (b) Identify the anomeric carbon atoms, and indicate whether each is α or β.
- (c) State the numbers of the carbon atoms that form glycosidic linkages between monosaccharide A and monosaccharide B.
- (d) State the numbers of the carbon atoms that form glycosidic linkages between monosaccharide B and monosaccharide C.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work. don't give Ai generated solution....give correct solution
Biochemistry
What is the process of "transamination" in either the muscles or the liver, that involves keto acid or glutamic acid?
Please explain how the steps work. Thank you!
Biochemistry
Please help. Thank you
What is the importance of glutamic acid in the metabolism of nitrogen from amino acids? (we know therole; it’s used to remove the nitrogen from amino acids so that the remaining carbon skeleton can bebroken down by the “usual” pathways, but what is the important, unique role that only glutamicacid/glutamate can do?)
Chapter 20 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 20.1 - Classify the following monosaccharides as an...Ch. 20.1 - Prob. 20.2PCh. 20.2 - Prob. 20.3PCh. 20.2 - Prob. 20.4PCh. 20.2 - Prob. 20.6PCh. 20.3 - D-Talose, a constituent of certain antibiotics,...Ch. 20.3 - Prob. 20.8PCh. 20.3 - Draw the structure that completes the mutarotation...Ch. 20.4 - Prob. 20.10KCPCh. 20.4 - Prob. 20.11P
Ch. 20.4 - Prob. 20.12PCh. 20.4 - Prob. 20.13PCh. 20.4 - Prob. 20.1CIAPCh. 20.4 - Prob. 20.2CIAPCh. 20.4 - All cells in your body contain glycoproteins...Ch. 20.5 - Draw the structure of the and anomers that...Ch. 20.6 - Prob. 20.15PCh. 20.6 - Prob. 20.16PCh. 20.6 - Prob. 20.17KCPCh. 20.7 - Prob. 20.4CIAPCh. 20.7 - Prob. 20.5CIAPCh. 20.7 - Prob. 20.6CIAPCh. 20.7 - Prob. 20.7CIAPCh. 20.7 - Prob. 20.18PCh. 20.7 - Prob. 20.19PCh. 20.7 - Prob. 20.8CIAPCh. 20.7 - Prob. 20.9CIAPCh. 20.7 - Prob. 20.10CIAPCh. 20 - During the digestion of starch from potatoes, the...Ch. 20 - Prob. 20.21UKCCh. 20 - Consider the trisaccharide A, B, C shown in...Ch. 20 - Hydrolysis of both glycosidic bonds in the...Ch. 20 - Prob. 20.24UKCCh. 20 - Are one or more of the disaccharides maltose,...Ch. 20 - Prob. 20.26UKCCh. 20 - Prob. 20.27UKCCh. 20 - Prob. 20.28APCh. 20 - What is the family-name ending for a sugar?Ch. 20 - Prob. 20.30APCh. 20 - Classify the four carbohydrates (a)(d) by...Ch. 20 - Prob. 20.32APCh. 20 - How many chiral carbon atoms are there in each of...Ch. 20 - Prob. 20.34APCh. 20 - Prob. 20.35APCh. 20 - Name four important monosaccharides and tell where...Ch. 20 - Prob. 20.37APCh. 20 - Prob. 20.38APCh. 20 - What is the structural relationship between...Ch. 20 - Prob. 20.40APCh. 20 - In Section 15.6, you saw that aldehydes react with...Ch. 20 - Sucrose and D-glucose rotate plane-polarized light...Ch. 20 - Prob. 20.43APCh. 20 - Prob. 20.44APCh. 20 - Prob. 20.45APCh. 20 - What is mutarotation? Do all chiral molecules do...Ch. 20 - What are anomers, and how do the anomers of a...Ch. 20 - What is the structural difference between the ...Ch. 20 - D-Gulose, an aldohexose isomer of glucose, has the...Ch. 20 - Prob. 20.50APCh. 20 - In its open-chain form, D-altrose has the...Ch. 20 - Prob. 20.52APCh. 20 - Prob. 20.53APCh. 20 - Prob. 20.54APCh. 20 - Prob. 20.55APCh. 20 - What is the structural difference between a...Ch. 20 - What are glycosides, and how can they be formed?Ch. 20 - Prob. 20.58APCh. 20 - Prob. 20.59APCh. 20 - Give the names of three important disaccharides....Ch. 20 - Lactose and maltose are reducing disaccharides,...Ch. 20 - Amylose (a form of starch) and cellulose are both...Ch. 20 - Prob. 20.63APCh. 20 - Prob. 20.64APCh. 20 - Prob. 20.65APCh. 20 - Gentiobiose, a rare disaccharide found in saffron,...Ch. 20 - Prob. 20.67APCh. 20 - Prob. 20.68APCh. 20 - Prob. 20.69APCh. 20 - Amylopectin (a form of starch) and glycogen are...Ch. 20 - What is the physiological purpose of starch in a...Ch. 20 - Prob. 20.72APCh. 20 - Prob. 20.73APCh. 20 - Prob. 20.74CPCh. 20 - Prob. 20.75CPCh. 20 - Prob. 20.76CPCh. 20 - Prob. 20.77CPCh. 20 - Prob. 20.78CPCh. 20 - Write the open-chain structure of the only...Ch. 20 - Prob. 20.80CPCh. 20 - Prob. 20.81CPCh. 20 - When a person cannot digest galactose, its reduced...Ch. 20 - Describe the differences between mono-, di-, and...Ch. 20 - Prob. 20.84CPCh. 20 - Prob. 20.85CPCh. 20 - Many people who are lactose intolerant can eat...Ch. 20 - Prob. 20.87GPCh. 20 - Prob. 20.88GPCh. 20 - Prob. 20.89GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Biochemistry Please help. Thank you When carbamyl phosphate is joined to L-ornathine, where does the energy for the reaction come from?arrow_forwardBiochemistry Question Please help. Thank you What is the function of glutamate dehydrogenase?arrow_forwardBiochemistry Question Please help. Thank you How and why does a high protein diet affect the enzymes of the urea cycle?arrow_forward
- Biochemistry What is the importance of the glucose-alanine cycle?arrow_forwardBiochemistry Assuming 2.5 molecules of ATP per oxidation of NADH/(H+) and 1.5molecules of ATP per oxidation of FADH2, how many ATP are produced per molecule of pyruvate? Please help. Thank youarrow_forward1. How would you explain the term ‘good food’? 2. How would you define Nutrition? 3. Nutrients are generally categorised into two forms. Discuss.arrow_forward
- Biochemistry Question. Please help solve. Thank you! Based upon knowledge of oxidation of bioorganic compounds and howmuch energy is released during their oxidation, rank the following, from most to least, with respect to how much energy would be produced from each during their oxidation. Explain your placement for each one.arrow_forwardBiochemistry Question.For the metabolism of amino acids what is the first step for theirbreakdown? Why is it necessary for this breakdown product to be transported to the liver? For the catabolism of the carbon backbone of these amino acids, there are 7 entry points into the “standard” metabolic pathways. List these 7 entry points and which amino acids are metabolized to these entry points. Please help. Thank you!arrow_forwardBiochemistry Question. Please help. Thank you. You are studying pyruvate utilization in mammals for ATP production under aerobic conditions and have synthesized pyruvate with Carbon #1 labelled with radioactive C14. After only one complete cycle of the TCA cycle, which of the TCA cycle intermediates would be labeled with C14? Explain your answer. Interestingly, you find C14 being excreted in the urine. How does it get there?arrow_forward
- Biochemistry question. Please help with. Thanks in advance For each of the enzymes listed below, explain what the enzyme does including function, names (or structures) of the substrate and products and the pathway(s) (if applicable) it is/are found in. (a) ATP synthetase (b) succinate dehydrogenase (c) isocitrate lyase (d) acetyl CoA carboxylase (e) isocitrate dehydrogenase (f) malate dehydrogenasearrow_forwardDraw and name each alcohol and classify it as primary, secondary, or tertiary. Explain your answer thoroughly.arrow_forwardDraw the product of each reaction. If there are multiple products, draw only the major product. Explain your answer thoroughly.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Metabolic Pathways; Author: Wisc-Online;https://www.youtube.com/watch?v=m61bQYio9ys;License: Standard Youtube License