
Campbell Biology
12th Edition
ISBN: 9780135188743
Author: Urry
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 9TYU
DRAW IT Draw Lewis dot structures for each hypothetical molecule shown below, using the correct number of valence electrons for each atom. Determine which molecule makes sense because each atom has a complete valence shell and each bond has the correct number of electrons. Explain what makes the other molecule nonsensical, considering the number of bonds each type of atom can make.
(a)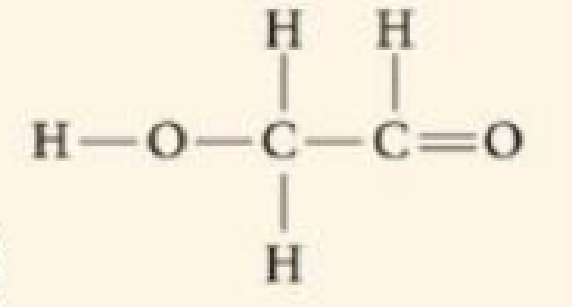
(b) 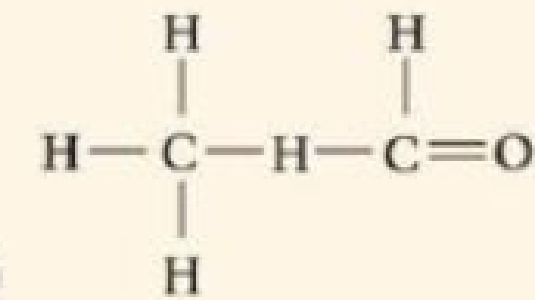
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1.)Draw 2,2‑dimethylbutane. Show all hydrogen atoms.
2.)Draw 3‑ethylhexanoic acid. Draw the structure in line‑bond form.
Structural isomers, like leucine and isoleucine, differ from each other in which of the following ways?
they have different numbers of atoms, but the same 3-dimensional arrangements of covalent bonds
they have different stoichiometric formulas, but the same number of covalent bonds
they have different atoms, but the same number of covalent bonds
they have different covalent bonds, but the same stoichiometric formulas
they have different numbers of atoms, and different 3-dimensional arrangements of covalent bonds
Compound A is a dipeptide, optically inactive. While compound B is a tripeptide, and optically active. Compound A is formed when compound C and compound D joined together by condensation reaction. Whereas monomers E and F are formed by modifying the compounds C and D. Polymer G is formed by the condensation reaction of monomers E and F.
Draw the possible structural formulae A, B,C,D,E,F and polymer G.
Label the peptide bond(s) for compounds A and B.
Pls name the compounds explain too
Chapter 2 Solutions
Campbell Biology
Ch. 2.1 - MAKE CONNECTIONS Explain how table salt has...Ch. 2.1 - Is a trace element an essential element? Explain.Ch. 2.1 - Prob. 3CCCh. 2.1 - MAKE CONNECTIONS Explain how natural selection...Ch. 2.2 - Prob. 1CCCh. 2.2 - A nitrogen atom has 7 protons, and the most common...Ch. 2.2 - Prob. 3CCCh. 2.2 - Prob. 4CCCh. 2.3 - Why does the structure H C = C H fail to make...Ch. 2.3 - What holds the atoms together in a crystal of...
Ch. 2.3 - What holds the atoms together in a crystal of...Ch. 2.4 - Prob. 1CCCh. 2.4 - Which type of chemical reaction, if any, occurs...Ch. 2.4 - WHAT IF? Write an equation that uses the products...Ch. 2 - Compare an element and a Compound.Ch. 2 - DRAW IT Draw the electron distribution diagrams...Ch. 2 - In terms of electron sharing between atoms,...Ch. 2 - What would happen to the concentration of products...Ch. 2 - Prob. 1TYUCh. 2 - Prob. 2TYUCh. 2 - The reactivity of an atom arises from (A) the...Ch. 2 - Which Statement is true of all atoms that are...Ch. 2 - Which of the following statements correctly...Ch. 2 - Prob. 6TYUCh. 2 - The atomic number of sulfur is 16. Sulfur combines...Ch. 2 - What coefficients must be placed in the following...Ch. 2 - DRAW IT Draw Lewis dot structures for each...Ch. 2 - EVOLUTION CONNECTION The percentages of naturally...Ch. 2 - SCIENTIFIC INQUIRY Female luna moths (Actias luna)...Ch. 2 - Prob. 12TYUCh. 2 - Prob. 13TYU
Additional Science Textbook Solutions
Find more solutions based on key concepts
A student moving out of a dormitory crouches in correct fashion to lift a heavy box of books. What prime movers...
HUMAN ANATOMY
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (6th Edition) - standalone book
1. The correct sequence of levels forming the structural hierarchy is
A. (a) organ, organ system, cellular, che...
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Convert the following structural formulas into condensed structures.arrow_forwardUse Frost Circles to complete the molecular orbital diagram for cyclooctatetrane. Label the bonding, non bonding, and anti bonding MO’s. If the molecule is planar, would it be aromatic, antiaromatic, or nonaromatic? If the molecule is nonplanar, would it be aromatic, antiaromatic, or nonaromatic?arrow_forwardIdentify the functional groups in the following molecule as pointed by arrow A and B, then C and Darrow_forward
- Consider nitrous acid, HNO2 (HONO).(a) Write a Lewis structure.(b) What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO2 molecule?(c) What is the hybridization on the internal oxygen and nitrogen atoms in HNO2?arrow_forwardConsider the given data (Solubility Tests, Solubility Group, Chemical Tests, and Functional Group) in the experiment. Based on the results of the solubility tests and chemical tests, what is the most probable structure of C4H6O2? Draw your answer. You may draw the compound using line-bond formula OR Lewis structure.arrow_forwardH₂C-C Ö: CH2 H3C — с O CH₂ + Clear The Lewis structure of acetone enolate ion, which has one other resonance structure, is shown. Complete the resonance structure by dragging bonds, charges, and electron lone pairs to their appropriate positions. Then check your answer.arrow_forward
- Compound P was discovered by a scientist. Compound P is a dipeptide, optically active and has the molecular formula C„H14N2O3. Compound P is formed when compound Q and compound R joined together by condensation reaction. While, monomers S and T are formed by modifying the compounds Q and R. Polymer U is formed by the condensation reaction of monomers S and T. Draw the possible structural formulae of compounds P, Q, R, S, T and U. Label the peptide bond(s) for compound P. Draw the possible structural formulae for repeating unit of polymer U. Please state the number of functional groups present in compound P.arrow_forwardplease determine the bond orders and compare the bond lengths of N2, N2-, and N2^2-species Using molecular orbital (MO) energy-level diagram,arrow_forwardIn an ammonia molecule, one nitrogen atom (atomic number = 7; 1s² 2s²2p³) forms covalent bonds with three hydrogen atoms. Draw a diagram of an ammonia molecule. Show all valence electrons, lone pairs, molecular geometry, and partial charges (assume sp³ hybridization).arrow_forward
- Compare the average N–O bond in NO3– to the average N–O bonds in NO2+ and NO2– (from above). Rank the three N–O bonds from weakest to strongest and from shortest to longest.arrow_forwardDiscuss whether the following statement is correct: “An ionic bond can, in principle, be thought of as a very polar covalent bond. Polar covalent bonds, then, fall somewhere between ionic bonds at one end of the spectrum and nonpolar covalent bonds at the other end.”arrow_forwardDetermine the chemical formula for the following molecules by counting the Carbon, Hydrogen and Oxygen atoms. Determine if the molecule is a carbohydrate by checking the ratio of atoms. 5. Ho C 1 H-C-OH H-C-OH H-C-OH CH₂OH 6. CH₂OH HO-C=0 [ H-C-OH H-C-OH 1 H-C-DH I H - COH I H-C-H I H Chemical formula Carbohydrate ? Chemical formula Carbohydrate ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license