
Concept explainers
Name the following:
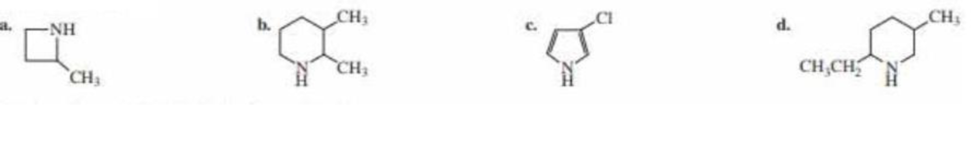
(a)
Interpretation:
The given chemical structure has to be named.
Concept Introduction:
The nomenclature of the chemical structures:
A molecular formula describes the total number and types of the atoms in a molecule, whereas chemical structures describe the arrangement of atoms in a compound.
International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules. The numbering starts from the side of substituent; if more than one substituent groups are present then these are cited alphabetically in the compound’s name.
Answer to Problem 21P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown below.
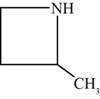
Figure 1
The compounds that comprise the saturated four membered rings are named as azetidines. The methyl substituent is present at the second carbon atom. Therefore, the name of the compound shown in figure is
(b)
Interpretation: The given chemical structure has to be named.
Concept Introduction:
The nomenclature of the chemical structures:
A molecular formula describes the total number and types of the atoms in a molecule, whereas chemical structures describe the arrangement of atoms in a compound.
International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules. The numbering starts from the side of substituent; if more than one substituent groups are present then these are cited alphabetically in the compound’s name.
Answer to Problem 21P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown below.
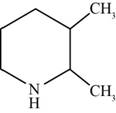
Figure 2
The compounds that comprise the saturated six membered rings with a nitrogen atom are named as piperidines. The methyl substituents are present at the second and third carbon atoms. Therefore, the name of the compound shown in figure 2 is
(c)
Interpretation: The given chemical structure has to be named.
Concept Introduction:
The nomenclature of the chemical structures:
A molecular formula describes the total number and types of the atoms in a molecule, whereas chemical structures describe the arrangement of atoms in a compound.
International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules. The numbering starts from the side of substituent; if more than one substituent groups are present then these are cited alphabetically in the compound’s name.
Answer to Problem 21P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown below.
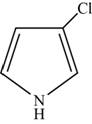
Figure 3
The compounds that comprise the unsaturated four membered rings with a nitrogen atom are named as pyrroles. The chlorine substituent is present at the third carbon atom. Therefore, the name of the compound shown in figure 3 is
(d)
Interpretation:
The given chemical structure has to be named.
Concept Introduction:
The nomenclature of the chemical structures:
A molecular formula describes the total number and types of the atoms in a molecule, whereas chemical structures describe the arrangement of atoms in a compound.
International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. IUPAC names are totally different from common names because IUPAC names are applied at international level and it comprises suffix, prefix, numbers and other priority rules. The numbering starts from the side of substituent; if more than one substituent groups are present then these are cited alphabetically in the compound’s name.
Answer to Problem 21P
The name of the given compound is
Explanation of Solution
The structure of the given compound is shown below.
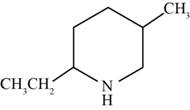
Figure 4
The compounds that comprise saturated six membered rings with a nitrogen atom are named as piperidines. The ethyl and methyl substituents are present at the second and fifth carbon atoms respectively. Therefore, the name of the compound shown in figure is
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry (8th Edition)
- The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Calculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forward
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forward
- In the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forwardThe degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forwardIn natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




