
Concept explainers
(a)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
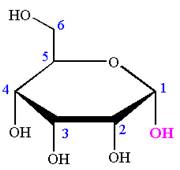
Explanation of Solution
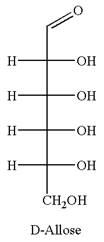
The given molecule is
The structure of
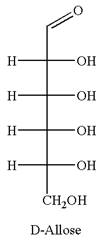
The name
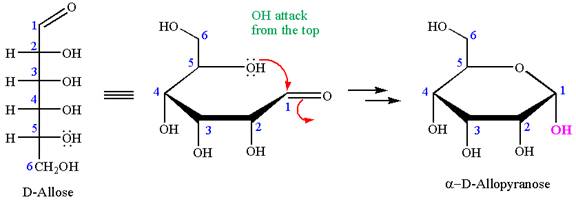
The Haworth projection of
(b)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
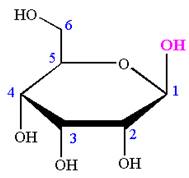
Explanation of Solution
The given molecule is
The structure of
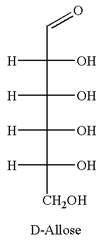
The name
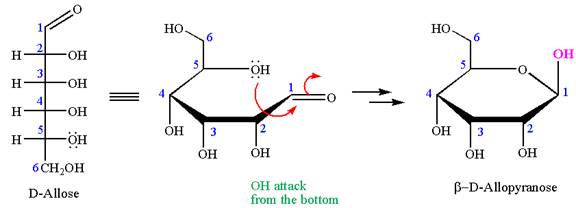
The Haworth projection of
(c)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
Explanation of Solution
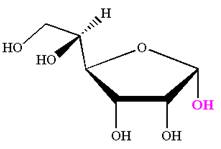
The given molecule is
The structure of
The name
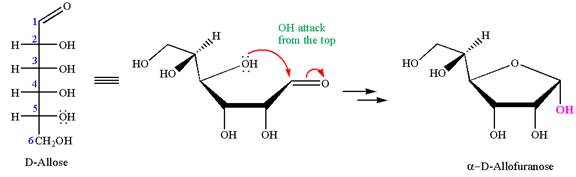
The Haworth projection of
(d)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
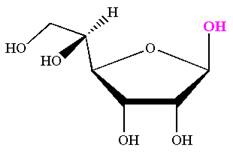
Explanation of Solution
The given molecule is
The structure of

The name
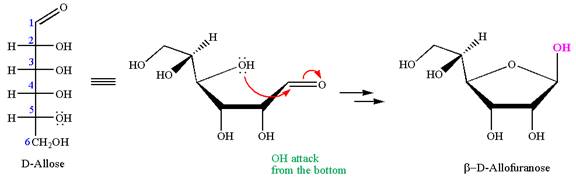
The Haworth projection of
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Please provide a detailed and clear explanation according to what question asked. Also, please provide a relevant diagram. Can ignore question ( i ) and ( ii ) if it violates the policy.arrow_forwardHelp with this question please......draw if possarrow_forwardDraw a curved arrow mechanism for the reaction. You can assume that all reactants and products are shown.arrow_forward
- Please provide a detailed and clear explanation according to what question asked. Also, please provide a relevant diagram.arrow_forwardThe saccharide shown here is present in some plant-derived foods. (a) Indicate the anomeric carbon atom(s) in this structure by drawing a circle around the atom(s) or by drawing an arrow pointing to the atom(s). (b) Would this saccharide give a positive result with Benedict’s reagent? Why or why not? (c) Would this saccharide give a positive result with Barfoed’s reagent? Why or why not? (d) Would this saccharide give a positive result with Seliwanoff’s reagent? Why or why not? (e) In a separate set of experiments, the saccharide solution was treated with a reagent that breaks glycosidic bonds. After this treatment, would any of the three assays give different results? Be sure to indicate which assay results would be different and give a reason.arrow_forward1. Which of the following is not true about halohydrin formation of alkenes? (a) Water acts as a nucleophile. (b) The water attacks syn to the halide. (c) Water attacks the more substituted carbon. (d) The reaction is often performed in DMSO solvent. alating marrow_forward
- Provide details with your answers.arrow_forwardGive a clear handwritten answer...arrow_forwardSaquinavir (trade name Incirase) belongs to a class of drugs called protease inhibitors, which are used to treat HIV. Locate all the stereogenic centers in the drug saquinavir. H. H. N. N. H. NH2 OH O: saquinavir Trade name: Invirase NH O:arrow_forward
- (a) p-Nitrobenzaldehyde is reactive toward nucleophilic additions than p- more methoxybenzaldehyde. Draw both of the molecular structures and explain the observation. (b) Cyclohexanone forms a cyanohydrin in good yield but 2,2,6-trimethylcyclohexanone does not. Draw both of the molecular structures and explain the observation.arrow_forwardClick the "draw structure" button to launch the drawing utility. What alkene is the major product formed from the following alkyl halide in an E1 reaction? CI The major product is: draw structure ...arrow_forwardGive detailed Solution with explanation (no need Handwritten answerarrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

