
(a)
Interpretation: The graph of concentration (y-axis) versus time (x-axis) has to be sketched.
Concept Introduction: The rate is a method for determining how much something changes over a predetermined period of time. The amount of reactant or product that changes per unit of time is the
(a)
Answer to Problem 108A
The graph of concentration (y-axis) versus time (x-axis) is as follows:
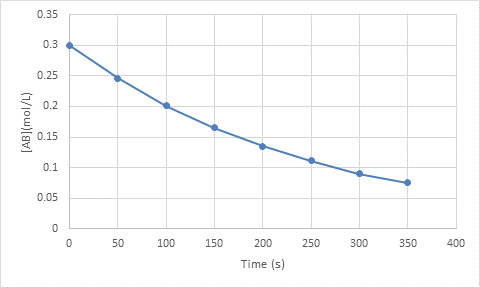
Explanation of Solution
The change in concentration of a substance with respect to unit time gives the rate of a reaction. The mathematical equation for the rate is given as follows:
The rate of the reaction is monitored by either the appearance of the product or the disappearance of the reactant over time.
(b)
Interpretation: The rate of the reaction at
Concept Introduction: The rate is a method for determining how much something changes over a predetermined period of time. The amount of reactant or product that changes per unit of time is the reaction rate. The change in concentration of a substance with respect to unit time gives the rate of a reaction. The mathematical equation for the rate is given as follows:
Here,
(b)
Answer to Problem 108A
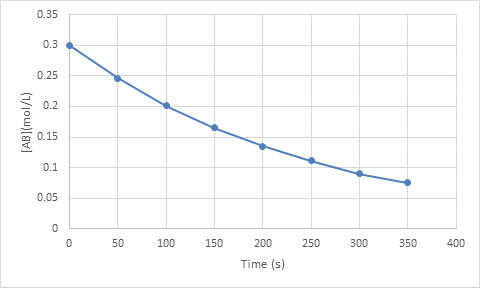
The rate of the reaction at
Explanation of Solution
Given information:
The initial concentration is
The given time is
The concentration at
The concentration at
The change in concentration of a substance with respect to unit time gives the rate of a reaction. The mathematical equation for the rate is given as follows:
Here,
The rate of the reaction is monitored by either the appearance of the product or the disappearance of the reactant over time.
To calculate the rate at
To calculate the rate at
Chapter 18 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





