
(a)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an
For an Example:
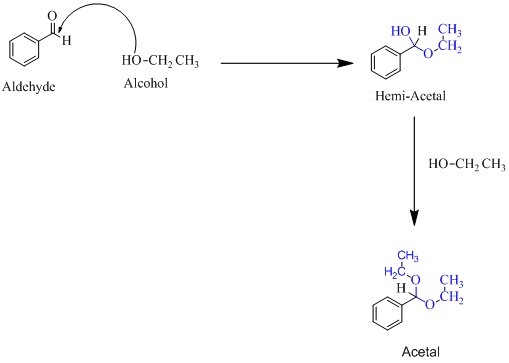
(b)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
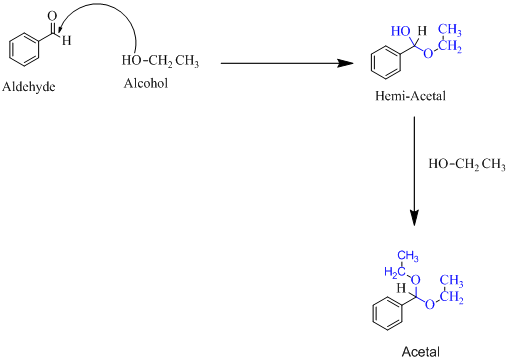
(c)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
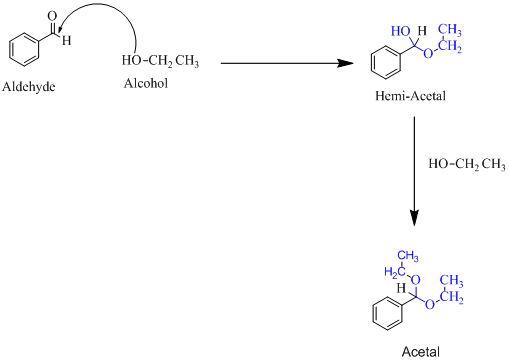
(d)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
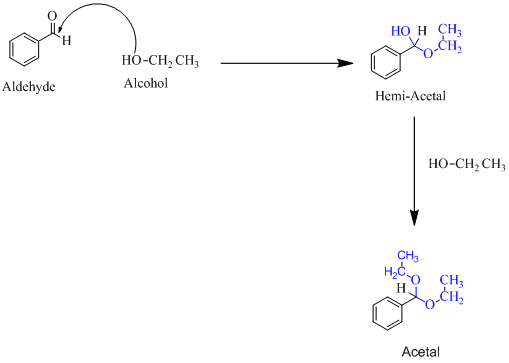
(e)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
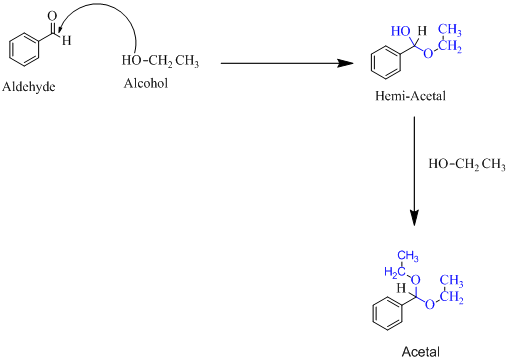
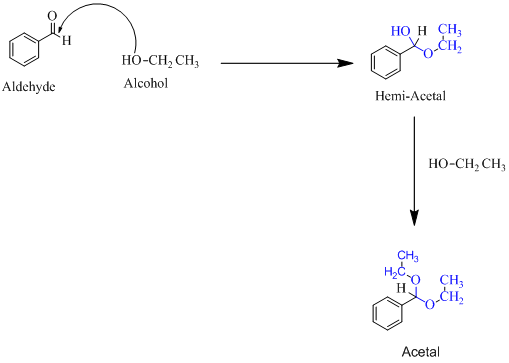
(f)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
Trending nowThis is a popular solution!

Chapter 17 Solutions
Introduction to General, Organic and Biochemistry
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning




