
(a)
Interpretation:
The structure of six-membered cyclic hemiacetal formed by 5-hydroxyhexanal should be drawn.
Concept Introduction:
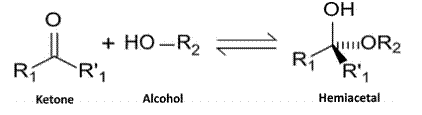
Hemiacetal is a molecule formed by the addition of an alcohol to an
Answer to Problem 17.71P
Explanation of Solution
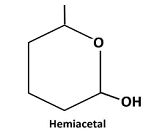
Reaction of the aldehyde group and the hydroxyl group forms a six-membered cyclic hemiacetal.
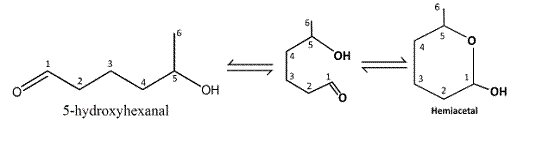
Therefore, the structure hemiacetal formed from 5-hydroxyhexanalis as follows:
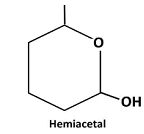
(b)
Interpretation:
The number of possible stereoisomers of 5-hydroxyhexanal should be determined.
Concept Introduction:
Stereoisomers are the compounds that are differ only in the spatial arrangements of their atoms. For a molecule with n stereoisomers, a maximum of
Answer to Problem 17.71P
5-hydroxyhexanal has 2 stereoisomers.
Explanation of Solution
For a molecule with n stereoisomers, a maximum of
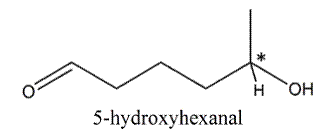
In 5-hydroxyhexanal, there is one stereocenter.
Therefore, number of stereoisomers can be calculated as follows:
Thus, number of stereoisomers will be 2.
(c)
Interpretation:
The number of possible stereoisomers of hemiacetal of 5-hydroxyhexanal should be calculated.
Concept Introduction:
Stereoisomers are the compounds that are differ only in the spatial arrangements of their atoms. For a molecule with n stereoisomers, a maximum of
Answer to Problem 17.71P
Hemiacetal of 5-hydroxyhexanal has 4 stereoisomers.
Explanation of Solution
For a molecule with n stereoisomers, a maximum of
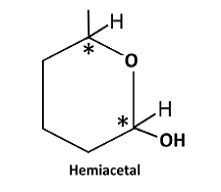
In hemiacetal of 5-hydroxyhexanal, there are two stereo centres.
Therefore, number of stereoisomers can be calculated as follows:
Thus, number of stereoisomers are 4.
Want to see more full solutions like this?
Chapter 17 Solutions
Introduction to General, Organic and Biochemistry
- Hi, I need help on my practice final, if you could explain how to solve it offer strategies and dumb it down that would be amazing. Detail helpsarrow_forwardBriefly explain the following paragraph: both the distortion of symmetry and the fact that the solid is diamagnetic indicate the existence of a Nb-Nb bond.arrow_forwardHi I need help on my practice final, If you could explain how to solve it, offer strategies, and dumb it down that would be amazing.arrow_forward
- -1 2 3 4 5 7 8 At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0635 s 2C1,0, (g) →2C1, (g)+50, (g) Suppose a vessel contains C1,0, at a concentration of 1.03 M. Calculate how long it takes for the concentration of C1,0, to decrease by 86.0%. You may assume no other reaction is important. Round your answer to 2 significant digits. e х th Earrow_forwardASAP....arrow_forwardNonearrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


