
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 43P
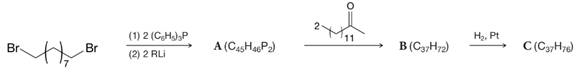
The structure of the sex pheromone (attractant) of the female tsetse fly has been confirmed by the following synthesis. Compound C appears to be identical to the natural pheromone in all respects (including the response of the male tsetse fly). Provide structures for A, B, and C.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Don't use ai to answer I will report you answer
Provide the correct common name for the compound shown here.
Ph
heat
heat
Chapter 16 Solutions
Organic Chemistry
Ch. 16 - PRACTICE PROBLEM 16.1 (a) Give IUPAC substitutive...Ch. 16 - Prob. 2PPCh. 16 - Prob. 3PPCh. 16 - Practice Problem 16.4
Provide the reagents and...Ch. 16 - Prob. 5PPCh. 16 - Prob. 6PPCh. 16 - Prob. 7PPCh. 16 - Prob. 8PPCh. 16 - Prob. 9PPCh. 16 - Practice Problem 16.10
Shown below is the...
Ch. 16 - Prob. 11PPCh. 16 - Practice Problem 16.12
What product would be...Ch. 16 - Prob. 13PPCh. 16 - Practice Problem 16.14
Dihydropyran reacts readily...Ch. 16 - Practice Problem 16.15 Show how you might use...Ch. 16 - Practice Problem 16.16 (a) Show how you might...Ch. 16 - Practice Problem 16.17
In addition to...Ch. 16 - Practice Problem 16.18
Triphenylphosphine can be...Ch. 16 - Prob. 19PPCh. 16 - PRACTICE PROBLEM 16.20
Give the structure of the...Ch. 16 - PRACTICE PROBLEM 16.21 What would be the major...Ch. 16 - Prob. 22PCh. 16 - 16.23 Write structural formulas for the products...Ch. 16 - Give structural formulas for the products formed...Ch. 16 - 16.25 What products would be obtained when...Ch. 16 - Predict the major organic product from each of the...Ch. 16 - 16.27 Predict the major product from each of the...Ch. 16 - 16.28 Predict the major product from each of the...Ch. 16 - Prob. 29PCh. 16 - 16.30 Write detailed mechanisms for each of the...Ch. 16 - Prob. 31PCh. 16 - Prob. 32PCh. 16 - Show how you would convert benzaldehyde into each...Ch. 16 - 16.34 Show how ethyl phenyl ketone could be...Ch. 16 - Show how benzaldehyde could be synthesized from...Ch. 16 - Give structures for compounds AE. Cyclohexanol...Ch. 16 - Prob. 37PCh. 16 - Prob. 38PCh. 16 - Prob. 39PCh. 16 - Prob. 40PCh. 16 - Prob. 41PCh. 16 - Prob. 42PCh. 16 - 16.43 The structure of the sex pheromone...Ch. 16 - Provide reagents that would accomplish each of the...Ch. 16 - Write a detailed mechanism for the following...Ch. 16 - Prob. 46PCh. 16 - Dutch elm disease is caused by a fungus...Ch. 16 - Prob. 48PCh. 16 - Compounds W and X are isomers; they have the...Ch. 16 - Compounds Y and Z are isomers with the molecular...Ch. 16 - Compound A (C9H18O) forms a phenylhydrazone, but...Ch. 16 - Compound B (C8H12O2) shows a strong carbonyl...Ch. 16 - Prob. 53PCh. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - (a) What would be the frequencies of the two...Ch. 16 - Prob. 57PCh. 16 - Prob. LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q8. Perform the calculation to the correct number of significant figures.
a) 0.121
b) 0.12
c) 0.12131
d) 0.121...
Chemistry: A Molecular Approach (4th Edition)
Checkpoint 7:
What is the relationship between reactants and products in a chemical reaction?
Principles of Anatomy and Physiology
Describe the 1H NMR spectrum you would expect for each of the following compounds, indicating the relative posi...
Organic Chemistry (8th Edition)
10.71 Identify each of the following as an acid or a base: (10.1)
H2SO4
RbOH
Ca(OH)2
HI
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
All of the following terms can appropriately describe humans except: a. primary consumer b. autotroph c. hetero...
Human Biology: Concepts and Current Issues (8th Edition)
Assume that water sinking in the North Atlantic near Greenland takes 1000 years to resurface in the Indian Ocea...
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY