
(a)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate two signals in
Explanation of Solution
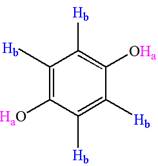
The structure of the given compound is
The given compound has a plane of symmetry and thus has two chemically distinct protons indicated as
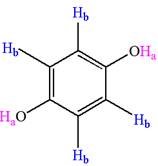
It is determined that the given molecule has two signals in
(b)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate five signals in
Explanation of Solution
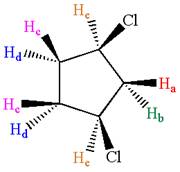
The structure of the given compound is
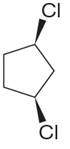
The protons cis to chlorine atoms and those trans to chlorine atoms are diastereotropic. Thus, the compound has five chemically distinct protons indicated as
It is determined that the given molecule has five signals in
(c)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate eight signals in
Explanation of Solution
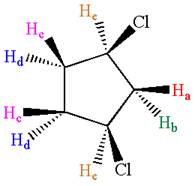
The structure of the given compound is
As the ring has two different substituents, all the protons are chemically non-equivalent. Thus, the compound has eight chemically distinct protons indicated as
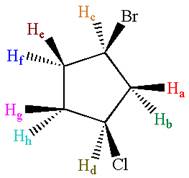
It is determined that the given molecule has eight signals in
(d)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate four signals in
Explanation of Solution
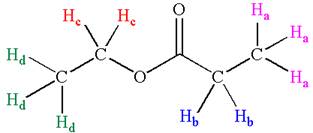
The structure of the given compound is
In the given compounds, both ethyl groups are in different chemical environment. One is attached to oxygen and another to carbonyl carbon. Thus the compound has four chemically distinct protons indicated as
It is determined that the given molecule has four signals in
(e)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate two signals in
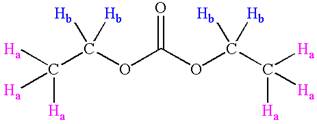
Explanation of Solution
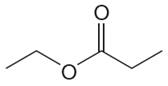
The structure of the given compound is
In the given compounds, both ethyl groups are in the same chemical environment. Thus, the compound has two chemically distinct protons indicated as
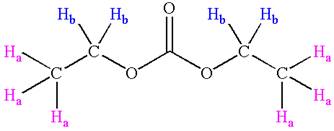
It is determined that the given molecule has two signals in
(f)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate three signals in
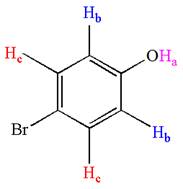
Explanation of Solution
The structure of the given compound is
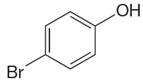
As the molecule is para disubstituted, it has a plane of symmetry passing through
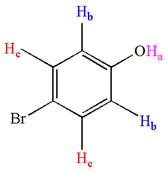
It is determined that the given molecule has three signals in
(g)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate five signals in
Explanation of Solution
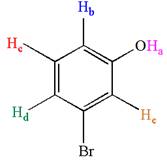
The structure of the given compound is
The molecule is meta disubstituted with different substituents and has no plane of symmetry; thus, all protons are chemically non-equivalent. Hence the compound has five chemically distinct protons indicated as
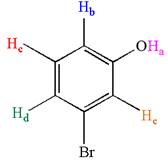
It is determined that the given molecule has five signals in
(h)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate five signals in
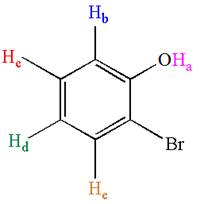
Explanation of Solution
The structure of the given compound is
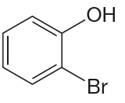
The molecule is ortho disubstituted with different substituents and has no plane of symmetry; thus, all protons are chemically non-equivalent. Hence the compound has five chemically distinct protons indicated as
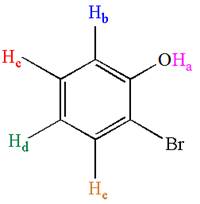
It is determined that the given molecule has five signals in
(i)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate six signals in
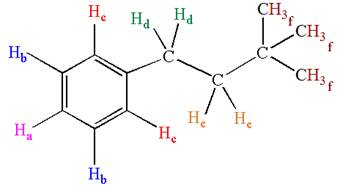
Explanation of Solution
The structure of the given compound is
The molecule is monosubstituted benzene; thus, there are three types of aromatic protons. The three methyl protons are in same chemical environment; thus they are identical. Hence the compound has six chemically distinct protons indicated as

It is determined that the given molecule has six signals in
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- So, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forwardDraw a mental model for calcium chloride mixed with sodium phosphatearrow_forward
- here is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forwardDrawing of 3-fluro-2methylphenolarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





